MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 3. ROOTS
A. Introduction
It is necessary to be familiar with the intrinsic structure of roots to recognise changes in root structure due to mycorrhizal associations. This section focuses on root structures with the potential to influence mycorrhizal development, as explained below. Additional information about roots can be found in plant anatomy and plant nutrition texts. The terminology used is explained in the glossary below and microscopy methods used to produce images introduced in Section 10.
| Topic | Major Sources |
| Images | Brundrett et al. 1990, Brundrett et al. 1996 |
| Diagrams and definitions | Brundrett et al. 1996 |
 |
WHAT ARE ROOTS? Roots are equal in importance to leaves as the life support system for plants and thus for all life in terrestrial ecosystems |
 |
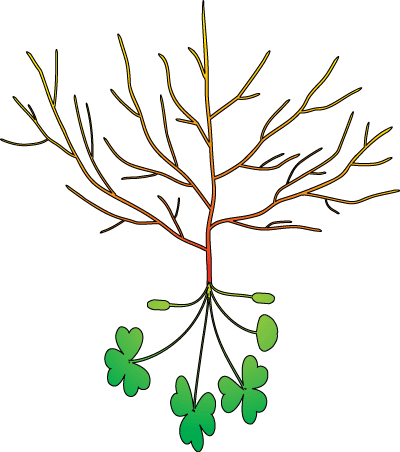
Roots are:
|
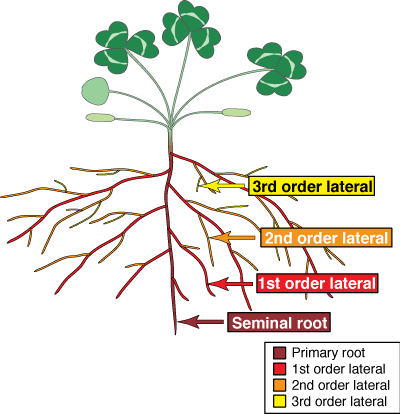
B. Root Systems
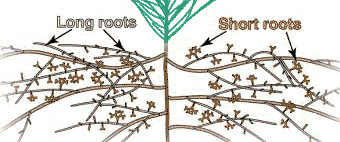
The recognition of different types of roots is important because they have different functions. Most plants produce one or more orders of lateral root branches that vary in thickness, branching patterns, growth rates, capacity for secondary growth, lifespan, structural features, etc. These variations will influence the capacities of roots to obtain water and nutrients, support mycorrhizal associations and survive adverse conditions. Higher order lateral roots are generally thinner, shorter and don't live as long as those of lower orders.
 |
Types of roots:
|
1. Root System Diversity
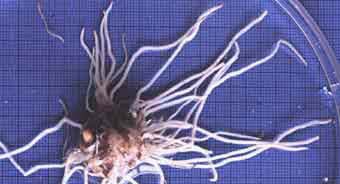
There are differences between plant species in the proportion of relatively fine or coarse roots they produce. Examples of plants with fine or coarse root systems are shown below. The nature of plant's root systems is related to their capacity of to grow without mycorrhizal associations. Plants may also have distinctive root branching patterns, such as the root clusters of some nonmycorrhizal (NM) plants.
It is difficult to identify roots with arbuscular mycorrhizas (VAM) by superficial examination. Species with ectomycorrhizal (ECM) associations typically have short, swollen lateral roots that may be visible without a microscope. However, some plants with VAM associations have dimorphic (beaded) roots (e.g. Acer and Ulmus, and Podocarpus). Thus, it is necessary to examine roots internally to identify association types, as explained in Section 10.
|
|
 |
Coarse root system of Arisaema atrorubens for comparison with the fine root system below. These thick, relatively unbranched roots with few root hairs is considered to be highly dependant on mycorrhizas (Brundrett & Kendrick 1988).
|
 |
Deciduous forest plant with fine roots (Geranium robertianum). This species with highly branched roots with long root hairs is considered to have a low requirement for mycorrhizas.
|
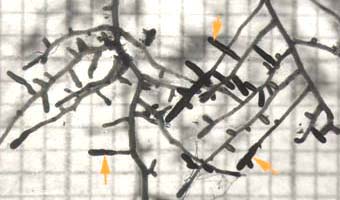 |
Ectomycorrhizal short roots (arrows) of birch (Betula alleghaniensis), an angiosperm tree. The mycorrhizal roots are thicker than other laterals of the same order due to mantle hyphae on the surface and epidermal cell enlargement in the Hartig net.
|
 |
Pine species with ectomycorrhizal associations have distinctive short, lateral roots with equal (dichotomous) branches. |
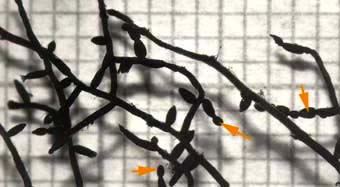 |
Root system of maple (Acer saccharum). This tree has short lateral roots with constrictions that are called beads (arrows). These result from constrictions when root growth stops as explained below.
|
 |
Cluster roots produced by a nonmycorrhizal plant. This is an Australian a species of Hakea in the Proteaceae family. The role of these roots is explained in Section 6.
|
C. Root Growth
Plants must produce new roots to grow larger, to explore new volumes of soil to acquire nutrients, and to replace old roots. Young roots are responsible for most direct nutrient uptake (Marschner 1986). Mycorrhizal formation by both ECM and VAM fungi also requires young roots. Young roots can be recognised by observing the distance of xylem and endodermis cell maturation from the root tip. Roots which have stopped growing have mature xylem vessels at their apex and may also have a suberised (metacutinized) root cap. These features are readily apparent after roots have been cleared and stained.
Root tissues are produced by cell division in the root apex and cell expansion in subapical regions. The apical meristem produces new root cap cells in an outward direction and new root cells in an inward direction, as shown in the diagram below, where root cell maturation is represented by increasing colour intensity and root tissues by different colours.
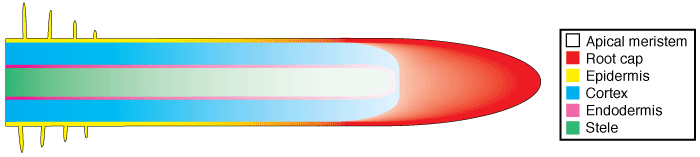 |
Most of what we know about plants comes from scientific studies of crops selected from weedy ancestors for rapid growth in highly fertile soils. However, this information may not be relevant to plants in natural ecosystems. Crop plants typically have roots which elongate 1 cm or more in a day (Russell 1977), while roots of plants from in a natural ecosystem may grow 1 mm or less a day (Brundrett & Kendrick 1990).
- Crop plants tend to be annuals with a relatively "soft" fine roots that only lasts a few weeks or months.
- Plants in natural ecosystems often produce perennial roots by investing in a coarse well-built root system.
- There is a trade-off between root lifespan with its associated features and the capacity of roots to acquire nutrients directly.
D. Root Structures
These images introduce the most important root structures people who work with mycorrhizas are likely to encounter. Most of these images were taken during studies of the roots and mycorrhizas of plants and trees in Canadian forests (Brundrett & Kendrick 1988, Brundrett et al. 1990, 1991).
|
|
|
|
1. Root Tips
|
The growing tip of roots is protected by a root cap consisting of concentric layers of cells surrounding the apical meristem where new root cells are produced. The surface of the root cap of growing roots is often covered by a thick layer of mucilage (Rougier & Chaboud 1985). When roots stop growing the root cap may be protected by suberisation of its outermost cells, as is shown below. These metacutinised root tips would generally not be produced by annual species such as crop plants, but are commonly produced by perennial plants such as trees (Romberger 1963, Brundrett & Kendrick 1988). |
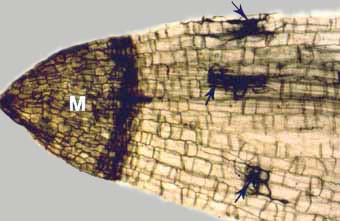 |
Low-magnification microscope view of a perennial woodland plant (Smilacina racemosa) with a metacutinised root cap (M) and mycorrhizal fungus hyphae (arrows). Note how dark-staining suberin in the root cap functions as an extension of the exodermis to completely encase the root for protection during periods of inactivity. Cleared root stained with chlorazol black E. |
 |
Longitudinal section of a sugar maple (Acer saccharum) root showing constrictions (beads) caused by root cap metacutinization (arrows) when roots resume growth. A Low magnification view of these roots is shown above.
|
2. The Epidermis and Root Hairs
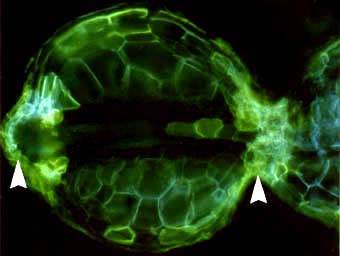
 |
The epidermis is the outmost layer of roots that functions as the interface between plants and the soil. Cell walls of epidermal cells may be lignified, or suberised, or be relatively unmodified. Cells of the epidermis of young roots secrete mucilages (Rougier & Chaboud 1985). Epidermal cells often have narrow outgrowths that extend between soil particles called root hairs. Root hairs may be long or short, dense, sparse, or absent altogether (Peterson & Farquhar 1996). Root hairs facilitate mineral nutrient uptake by increasing the surface area of roots (Marschner 1995, Lambers et al. 2008). There is a correlation between the degree of root hair production by plants and their requirement for mycorrhizas, as explained in Section 6 and Section 7. |
3. The Exodermis or Hypodermis
Cells of the surface layers of roots are often highly specialised. The root cell layer under the epidermis is called a hypodermis if it is relatively unmodified or an exodermis if it has suberised cell walls (Peterson 1988, Ma & Peterson 2003). Suberin is a hydrophobic mixture of lipids and phenolics deposited in the walls of some plant cells (Kolattukudy 1984). The exodermis is often similar in structure to the endodermis of roots, with Casparian bands and suberin lamellae. Many species have a suberised exodermis, which is thought to function as a permeability barrier and to help defend the root from parasites and adverse soil conditions (Brundrett & Kendrick 1988, Perumalla et al. 1990, Brundrett 2002, Ma & Peterson 2003). In some cases, the exodermis has two cell types - long and short cells (called a dimorphic exodermis - Shishkoff 1987). In these roots, the short cells have less suberin in their walls and are used as passage cells by mycorrhizal fungus hyphae.
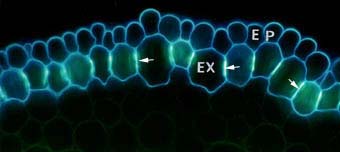 |
Cross section of an onion (Allium cepa) root showing modified cell walls of exodermal (EX) and epidermal (EP) cells. Exodermal cells have Casparian bands (arrows) in their radial walls and suberin lamellae.
|
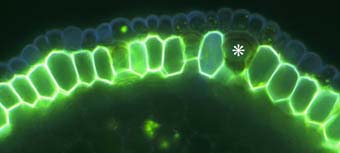 |
Close-up of the outer cell layers of an ash tree (Fraxinus) root showing suberin lamellae in exodermal cells. Note the short (passage) cell (*) without suberin lamellae.
|
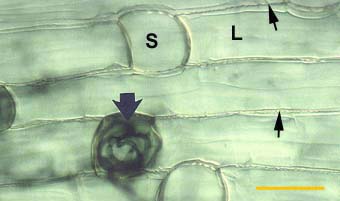 |
Root of Smilacina racemosa (a Canadian plant) with a dimorphic exodermis with alternating long (L) and short (S) cells. Casparian bands are seen as wavy lines between cells (thin arrows). A short cell contains hyphae for the entry point of a VAM colony (thick arrow).
|
4. Air Spaces
Plants which are adapted for growth in habitats where soils are often waterlogged typically have large air spaces in their roots (Armstrong 1979). These structures would greatly reduce the habitat available for VAM fungi in these roots. Narrow air channels occur in the roots of many species that grow in moist or dry soil.
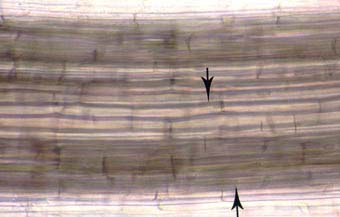 |
Intercellular air channels (arrows) in a leek (Allium porrum) root. These channels run continuously from the apex to the base of roots and influence mycorrhizal development if they are present in roots.
|
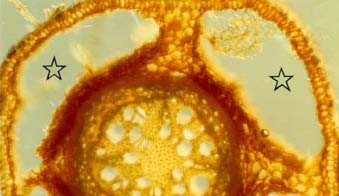 |
Cross-section of a willow (Salix nigra) root from wet soil with four large air spaces (stars) occupying most of the cortex. This root is in an early stage of secondary growth.
|
5. The Endodermis and Vascular Tissue
Conducting elements, which consist of xylem and phloem, occur within the vascular cylinder (stele) in the centre of roots. The vascular cylinder also contains other less-specialised cells and is enclosed by the endodermis. Both xylem and phloem are made of long narrow cells that are connected by their ends to form continuous networks of "plumbing". These networks interconnect plant organs.
- Xylem cells are dead at maturity and have thick, strong (lignified) cell walls. They transport water containing minerals and other solutes primarily towards the shoot.
- Phloem cells are alive at maturity and have thin walls. They transport metabolites, especially sugars resulting from photosynthesis, primarily from the leaves to the rest of the plant.
- The endodermis is cylinder of cells with suberised walls surrounding the stele and is thought to be a barrier to solute transport in the apoplast (cell wall space) (Clarkson & Robards 1975). The endodermis likely has an important role in regulating exchange processes in mycorrhizal associations as these fungi do not cross into the stele, so are confined to root spaces where the availability of resources is regulated by the endodermis.
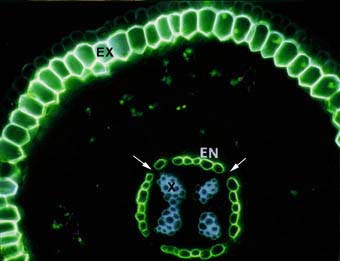 |
Cross section of an ash tree Fraxinus sp. root showing intense yellow fluorescence of suberin lamellae in the exodermis (EX) and endodermis (EN). Some endodermal cells are without suberin lamellae (arrows). These are called passage cells. Xylem cells (X), the exodermis (EX) and lipids in VAM fungus hyphae in the cortex can also be seen.
|
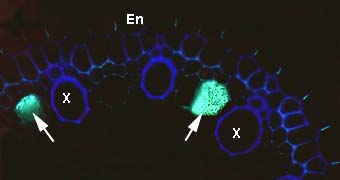 |
Phloem sieve tube elements in a root cross section. This image shows callus on the sieve plates which occur between longitudinally connected phloem cells. Endodermal (En) and xylem cells (X) are also visible.
|
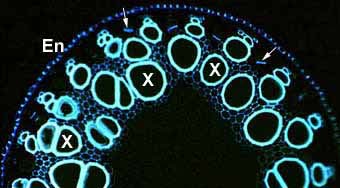 |
Numerous large xylem vessels (X) in a thick, low-order lateral asparagus root. Other smaller lignified cells are present in the stele, as are phloem sieve tube elements (arrows) and the endodermis (En).
|
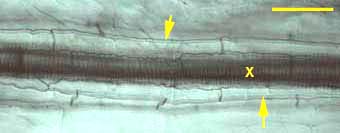 |
Endodermal Casparian bands (arrows) and longitudinally connected xylem cells (X).
|
6. Other Structures You are Likely to See
Roots may contain cells with other types of modified walls or cell inclusions such as crystals or secondary metabolites. Secondary metabolites often provide colour to roots and may result in UV-induced autofluorescence. Many plants, especially ferns and gymnosperms, accumulate large amounts of dark brown phenolics called tannins. Pigments of other colours are also common. The natural pigmentation of roots can help us distinguish young roots from older roots, which are often darker in colour.
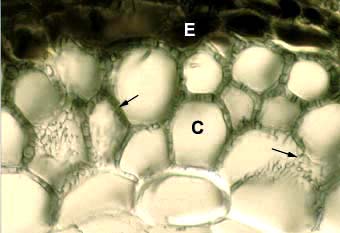 |
Accumulation of tannins in epidermal cells (E) visible in a cross section of a Tsuga canadensis (hemlock). Other structures in this ectomycorrhizal root are explained elsewhere.
|
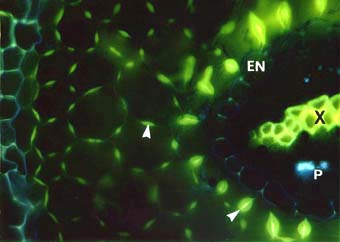 |
Cross section of a cedar (Thuja occidentalis) root with many lignified phi thickenings in cortex cell walls (arrows). Endodermal (EN), xylem (X) and phloem (P) cells are also visible.
|
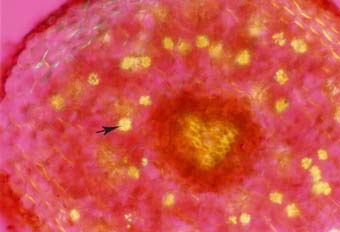 |
Primary root of black walnut (Juglans nigra) with clusters of crystals in many cells (arrow). These are probably calcium oxalate.
|
7. The Periderm and Secondary Growth
Some roots are genetically determined to have the capacity to undergo additional radial growth, but other roots do not. Only dicotyledon's and gymnosperms have this capacity and it is more likely to occur in thicker, lower-order lateral roots, especially if they have a long life span. This radial growth is called secondary growth to distinguish it from primary growth at the root apex.
Secondary growth occurs when new meristematic tissue forms in a ring around the vascular cylinder of roots and produces new xylem inwards and new phloem outwards. An outer bark layer made up of layers of suberised cells is also formed. Secondary growth eventually results in the loss of the cortex and epidermis of roots, so these roots cannot form mycorrhizas.
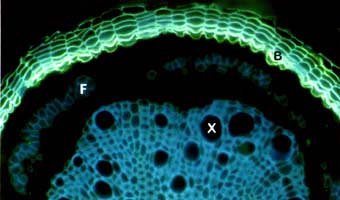 |
Fluorescent staining of suberised periderm (bark) cells (B) encasing a Quercus root after secondary growth. Blue autofluorescence of lignified xylem (X) and phloem fibre (F) cells are visible.
|
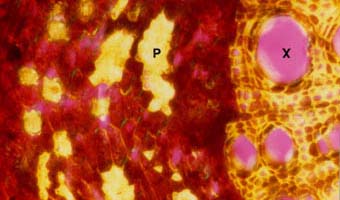 |
Strong refringence of thick cell walls of xylem (X) and phloem fibre (P) cells resulting from secondary growth in a Juglans nigra root.
|
E. Roles of Root Structural Features
As illustrated above, roots have diverse anatomical features. These features can be used to identify roots and have important roles. Possible roles of suberised walls in roots are listed below. Many structural features of roots influence their capacity to host and manage mycorrhizal associations, resulting from the long period of coevoluton of land plants and fungi as explained below.
1. Protection of the Root Surface
Roots of plants growing in natural ecosystems often have outer cell layers with a high degree of suberisation and/or lignification, unlike the crop plants we look at more often. These exodermal and epidermal cell wall modifications are permeability barriers and also strengthen and protect long-lived roots (Brundrett & Kendrick 1988). Chemical substances that accumulate in roots may also help to protect them from predators and parasites.
 |
|
2. Mycorrhiza Formation
The influence of root structural features on mycorrhizal formation is summarised in the table below. These interactions are considered further when mycorrhizal formation is discussed in the Sections 3 and 4.
Root Morphology Characteristics which Influence Mycorrhiza Formation (Brundrett 2004)
| Association | Anatomical feature | Influence on mycorrhizas |
| VAM | Cortex air channels | hyphal distribution and growth rates, arbuscule distribution |
| Epidermis and hypodermis structure | Appressorium position and path of root penetration | |
| ECM | Hypodermis structure | Hartig net type (epidermis or cortex) |
| Root growth rate | Efficiency of mycorrhiza formation |
Most mycorrhizas are formed by relatively fine high-order lateral roots. Coarse roots of monocotyledons do not have secondary growth, but may not form mycorrhizas if their primary cortex is heavily protected by suberised or lignified cells.
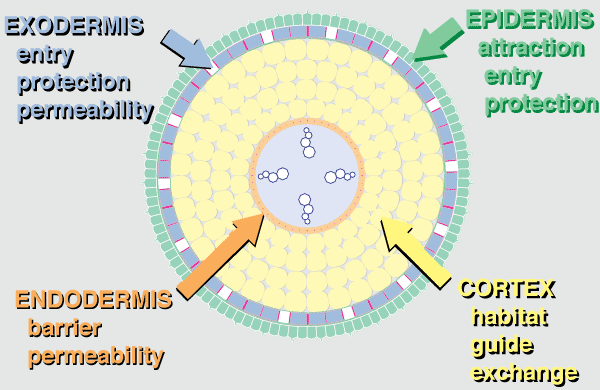 |
Anatomical evidence that roots evolved as habitats for mycorrhizal fungi. This diagrammatic summary illustrates typical features of angiosperm plants with VAM. |
Hover mouse over diagram for animated version.
3. Summary
- The epidermis is the first point of contact for mycorrhizal fungi. Epidermal cells usually produce long roots hairs in NM plants while those of mycorrhizal plants tend to be shorter. Mycorrhizas occur in epidermal cells in ECM associations of angiosperms.
- The exodermis is a permeability barrier and probably also provides greater structural strength, drought tolerance and reduced nutrient and water loss especially in long-lived roots.
- The cortex is the largest organ of primary roots and cortex cells contain little cytoplasm, unless occupied by a mycorrhizal fungus. With few exceptions (bryophytes, fern gametophytes, some orchids and myco-heterotrophs), plants only form mycorrhizas within a living root cortex or epidermis. The root cortex is also important for storage and transport, but these roles seem to require less volume, so cortex reduction typically follows the loss of mycorrhizas resulting in the narrow roots of many NM plants.
- The endodermis is the inner boundary of the cortex and is considered to have a major role in regulating nutrient transfer in all roots.
F. Terminology
A glossary of important terms used to describe root structures is provided below. You should refer to a plant anatomy text such as Esau (1977) for more information. Words in italics are defined elsewhere in the list.
A. SUBCELLULAR COMPONENTS
- Cell
- The basic component of plant organs, consisting of cytoplasm, organelles, vacuoles, etc, bounded by a plasma membrane.
- Cell wall
- Structure located outside the plasma membrane of most plant cells. It is primarily made of structural carbohydrates such as cellulose. Cell walls provide mechanical support and space for apoplastic transport of substances. They often contain secondary metabolites, suberin or lignin.
- Apoplast
- The cell wall space inside living plants is collectively known as the apoplast.
- Symplast
- The space inside living plant cells is collectively known as the symplast. The cytoplasm of adjacent plant cells is often connected by channels through the cell wall (plasmodesmata).
- Middle lamella
- A cell wall zone rich in the carbohydrate pectin connecting adjacent cells.
- Suberin
- This is a hydrophobic material, containing lipids and phenolics, which impregnates the cell walls of specialised cells (Kolattukudy 1984). Suberin is thought to prevent the passage of water and other materials in the apoplast.
- Suberin lamellae
- These are concentric layers of suberin deposited on the inner surface of cell walls and considered to function as barriers to microbial and solute penetration. These are most often found in endodermal or exodermal cells.
- Casparian band
- A specialised cell wall structure where suberin is deposited in a radial band. Cells with these structures are arranged in one or two cylinders within roots to form the endodermis and exodermis. These bands are thought to provide a barrier to apoplastic transport of solutes (Esau 1977, Clarkson & Robards 1975, Peterson 1988).
- Lignin
- A cell wall type that is impregnated by phenolic compounds. These walls are often considerably thickened to strengthen plant organs. Xylem cells and fibres are typically lignified, but other cells in the stele or cortex can have lignified walls.
- Phi thickenings
- These are localised deposits of lignified wall material which form a thickened ring in cortex cell walls (von Guttenberg 1968).
- Crystals
- Specialized root cells may contain crystals, along with mucilage, or other substances in their vacuole.
- Secondary metabolites
- Plant cells and cell walls often contain secondary metabolites (substances not required for metabolism). Phenolic compounds, including tannins (dark brown pigments) are especially common, but many other chemicals, including alkaloids, terpenes, flavanoids, etc., accumulate in roots of particular plant species. These may colour the root and result in uv-induced autofluorescence.
- Mucilage
- High molecular weigh, poorly diffusible substances actively secreted by root epidermal and root cap cells (Rougier & Chaboud 1985). These primarily consist of carbohydrates, but also may contain sloughed cells, enzymes, phenolic compounds, etc.
- Exudates
- Root exudates are defined as substance released into the substrate by healthy and intact plant roots (Rovira 1969). These include water, sugars, amino acids, etc.
B. CELLAR STRUCTURES
- Apex
- The root tip which is covered by a root cap (covering sheath) and secreted mucilage (water soluble polysaccharides which adhere to the root).
- Apical meristem
- The zone of dividing cells at the root apex which give rise to new cells in a growing root. Actively growing roots have gradients of maturing tissues away from the apical meristem.
- Epidermis
- The outermost layer of cells of the root, in direct contact with the soil. As the soil-root interface, the epidermis is an important site for nutrient uptake and the initiation of mycorrhizal associations.
- Root hair
- narrow cylindrical hair-like cell extension of an epidermal cell on the root surface. These may be long or short and provide a dense or sparse root covering. Root hairs increase root contact with the soil and are thought to have a role in water and nutrient uptake.
- Hypodermis
- The layer of cells below the epidermis is called a hypodermis if it is not suberised (Peterson 1988).
- Exodermis
- The hypodermis is called an exodermis if its cell walls contain a Casparian band and these cells often also have suberin lamellae (Peterson 1988). The exodermis is thought to reduce root permeability (to apoplastic flow) and increase resistance to pathogenic organisms, water loss, etc.
- Passage cells, short cells
- Small exodermal cells that remain unsuberised that are surrounded by longer suberised cells (long cells). In many plants, long and short cells alternate in a uniform pattern (called a dimorphic exodermis).
- Cortex
- The cell layers occurring between the epidermis and stele. Cortex cells typically have a large central vacuole used to store solutes and are the site of arbuscule formation in VAM associations.
- Aerenchyma
- Air spaces within plant organs. These can form between cells, or in the case of large spaces result from cell death. They often form continuous channels along the length or organs such as the root. The main role of aerenchyma is to provide gas exchange to cells in waterlogged soils (Armstrong 1979).
- Endodermis
- A cortex cell layer found in all roots, next to the vascular cylinder. The cell walls contain a Casparian band and may develop suberin lamellae (Esau 1965, Clarkson & Robards 1975).
- Intercellular space
- The spaces outside the root cells, often in the cortex at the junction of cells. These form longitudinal air channels in many roots, which can be seen by observing whole-living roots mounted in water. Air channels provide conduits for gas transport in waterlogged soils (Armstrong 1979) and influence VAM formation.
- Stele, vascular cylinder
- The zone internal to the endodermis which contains specialised tissue responsible for the transport of water and minerals to the shoot (xylem) or organic nutrients, such as photosynthetically fixed carbon, (phloem). Additional layers of xylem and phloem form radially during secondary growth and lateral root initiation also occurs in this zone. Xylem cells develop lignified walls and are dead when mature.
- Periderm
- The bark layer formed on the surface of roots or branches by secondary growth. Walls of periderm cells are strengthened by suberin and lignin deposits, which reduce their permeability and susceptibility to microbial activity and adverse soil conditions.
- Metacutinization
- This is the modification of dormant root tips by suberisation of one or more root cap cell layers (Romberger 1963). Inactive roots of many perennial plants develop a metacutinized apex, which functions as an extension of the exodermis, presumably for protection from adverse soil factors (Brundrett et al. 1990).
C. ORGANS AND ZONES
- Seminal root
- A root initiated by a germinating seed.
- Lateral roots
- Any root which grows from another root.
- Adventitious roots
- A root which arises from a stem.
- First order lateral roots
- Roots that arise from the seminal root or adventitious roots.
- Second and third order laterals, etc.
- Roots which arise from first order laterals which in turn may produce third order laterals, and so on. Higher order laterals may be categorised as feeder roots or fine roots (see below).
- Primary growth
- The initial growth of a plant organ caused directly by cell division in its apical meristem and cell enlargement in subapical regions.
- Secondary growth
- New growth activity which begins from mature cells in a plant organ. This normally results from radial enlargement of an organ by a new lateral meristem.
- Secondary roots, woody roots
- Roots, which develop a periderm and additional vascular tissue due to secondary growth. These would normally have a much longer lifespan than feeder roots and will not contain mycorrhizas if secondary growth has resulted in cortex loss.
- Coarse roots
- The "distributive" root system comprised of lower order roots, which is responsible for mechanical support and the transport of substances between fine roots and the shoot.
- Feeder roots, fine roots
- The fine, higher order lateral roots that are thought to be responsible for most nutrient and water uptake, as well as mycorrhiza formation.
- Brown roots, suberised roots, etc.
- These additional terms are sometimes use to designate old roots, woody roots, or roots with a suberised exodermis. These general terms are misleading and should not be used.
- Rhizosphere
- The zone surrounding roots where soil properties and microbial populations are influenced by root exudates.
- Rhizoplane
- The surface of the root and habitat for organisms which live in contact with the root.
Version 2 © Mark Brundrett - 1999, 2008