MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 10. METHODS FOR IDENTIFYING MYCORRHIZAS
A. How Images were Made
Most of the images of mycorrhizal associations presented on this website were made using relatively easy and inexpensive methods. Mycorrhizal images are of whole cleared roots or relatively thin cross sections of roots made by hand using a sharp razor blade. Staining and microscopy procedures used to make fungus hyphae visible are explained in detail in Subsection C, while more specific stains for plant structures are briefly introduced below.
| 1. Root Systems | |
 |
Low magnification images of root systems were taken with a camera and macro lens. This example is sand-binding roots in an Australian rush (Lyginia imberbis). |
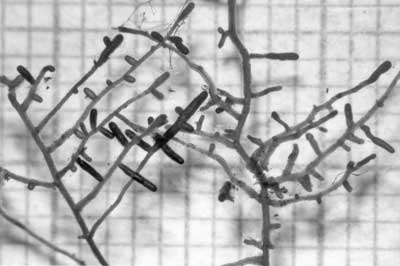 |
Relatively low magnification images of roots were taken with a dissecting microscope with a camera attachment. This example is of ECM short roots of birch (Betula alleghaniensis). |
| 2. Mycorrhizas | |
 |
A compound microscope allowed mycorrhizal structures within roots to be observed. These roots were first cleared in hot alkali to make them transparent and then stained with a dye (Chlorazal black E in most cases) that binds to fungal hyphae, as explained below. |
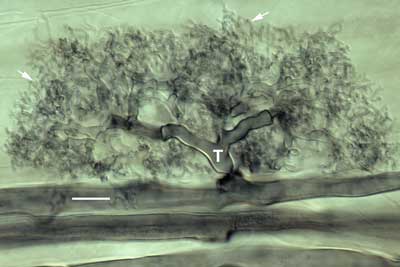 |
Interference contrast microscopy provided highly detailed images of mycorrhizal associations viewed at high magnification with a compound microscope. |
 |
Structural details of the Hartig net of ECM associations were observed using thin hand sections of roots. These sections were gently cleared in hot alkali, stained with Chlorazal black E and viewed with interference contrast microscopy (Brundrett et al 1990, 1996). Hand sections are made by cutting across root segments that were immobilised between pieces of laboratory film on a Petri dish lid (Frohlich 1984). |
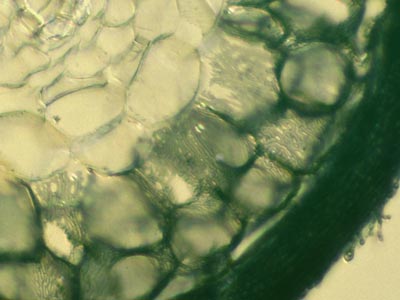
| 3. Root Anatomy | |
 |
In some cases root structures and mycorrhizal hyphae were observed using unstained hand sections under a fluorescence microscope with UV light. |
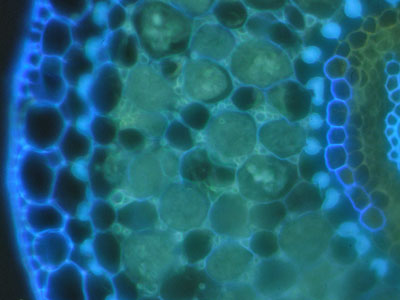
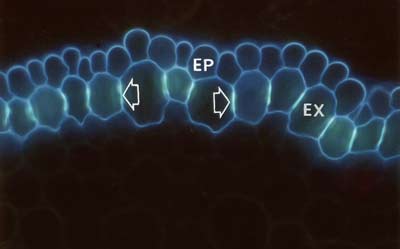 |
Suberin and lignin in hand sections of roots was stained with the fluorescent alkaloid Berberine with Aniline blue counterstaining and observed under a fluorescence microscope (Brundrett et al. 1988). |
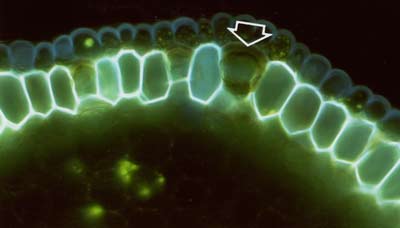 |
The lipid stain Fluorol was also applied to hand sections of roots to reveal suberin in exodermal and endodermal cells in fluorescence microscope images (Brundrett et al 1991). |
B. Safety Information
![]() It is the responsibility of all scientists and educators who use chemicals to obtain current safety information on materials which they use themselves or ask others to use. Mycorrhizal scientists routinely use chemicals which are considered to be dangerous. Safety information is provided by the suppliers of chemicals and the sources listed below.
It is the responsibility of all scientists and educators who use chemicals to obtain current safety information on materials which they use themselves or ask others to use. Mycorrhizal scientists routinely use chemicals which are considered to be dangerous. Safety information is provided by the suppliers of chemicals and the sources listed below.
Examples of Substances Considered to be Dangerous
|
Examples of sources of information |
C. Clearing and Staining Mycorrhizal Roots
The following sections are excerpts from the book: Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. 1996. Working with Mycorrhizas in Forestry and Agriculture (Chapter 4.2, pp. 179-183). See Vierheilig et al. (2005) for a review of all staining techniques for mycorrhizal fungi in roots.
Diagrams on this page are also available as pdf files to download.
Equipment and Reagents
|
Introduction
Structures produced by VAM fungi are invisible in fresh roots because internal structures are obscured by the natural pigments and cell contents within roots. While whole ECM roots can often be identified by observation with a dissecting microscope, internal details of these associations are revealed by removing pigments in roots cells and mantle hyphae. Clearing procedures that use chemical agents such as hot alkali to remove cell contents and cell wall pigments, are a valuable method for viewing internal features in plant tissues (Gardner 1975). Fungal structures in plant tissues can be observed by the use of stains which bond to fungal hyphae without much background staining of the cleared plant material. Stains such as Trypan blue, Chlorazol black E (CBE) in lactoglycerol, or ink in vinegar are used to stain mycorrhizal structures that were cleared by heating in KOH (Bevege 1968, Phillips & Hayman 1970, Kormanik & McGraw 1982, Brundrett et al. 1984, Vierheilig et al. 1998). This procedure is outlined below.
Safety warnings!!!
|

 |
1. Root samplesClearing and staining procedures require root samples that have been washed free of soil. It is imperative that KOH or staining solution volumes are sufficient for the amount of roots being processed and that roots are not tightly clumped together - for uniform contact with solutions. It is often best to chop roots into 2-4 cm long segments before clearing them. It may be necessary to sub- divide or sub-sample large volumes of roots to obtain good results. The fine roots of woody plants can also be separated from coarse roots, after determining their proportion of the total root system. |


 |
2. Clearing roots with KOH
|



 |
3. Staining roots with Chlorazol black E, Trypan blue, or Ink
|


 |
4. Working with darkly pigmented roots
|
 |
5. Alternative methods
|
 |
6. Sample storage and slide preparation
|
D. Quantifying Mycorrhizal Roots
Comprehensive instructions for processing root samples to detect and quantify mycorrhizas are published in manuals which should be consulted for further information (Brundrett et al. 1994, 1996). A brief summary of these methods is outlined below (after Chapter 4.3 in Brundrett et al. 1996).
Mycorrhizal studies often require estimates of the proportion of roots in a sample that contain mycorrhizal structures. After clearing and staining them, root length can be measured simultaneously with mycorrhizal colonization by a gridline intersection procedure (Giovannetti & Mosse 1980), or separately by viewing slides with a compound microscope (McGonigle et al. 1990).
The length of mycorrhizal roots present in a sample should be presented along with data on the proportion (%) of root length occupied by these fungi, because mycorrhizal root length is more directly correlated with association costs/benefits and inoculum production by the fungus. Root-length data can be used to calculate root production (growth) rates, root densities (within a volume of soil) and specific root lengths (root length/weight ratios) which provide valuable information about the capacity of roots to obtain water or nutrients from soils and their ability to form mycorrhizal associations.
- Analysis of colonisation data
- Data on mycorrhizal colonization of roots, and the distribution of fungal propagules such as spores, is often highly variable and/or has a non-normal frequency distribution (St. John & Hunt 1983, Friese & Koske 1991). Thus, statistical analysis of such data will usually require data to be transformed, or the use of non-parametric statistics.
Equipment
|
1. Roots with Arbuscular Mycorrhizas
- The most frequently used root measuring procedure is a modification of the grid line intersect method (Newman 1966, Tennant 1975, Giovannetti & Mosse 1980), in which roots are randomly dispersed in a 9-cm diameter Petri plate with grid lines as shown below. The observer scans along these grid lines with a dissecting microscope to quantify intersections between grid lines and roots — which are designated as either colonised or nonmycorrhizal.
- The proportion of root length that is mycorrhizal and total root length can then be calculated from a conversion factor derived from the total length of grid lines and the area of the dish (Newman 1966, Tennant 1975). If a 11/14 cm (approx. 1/2 inch) grid is used the number of intersects will provide values of mycorrhizal and non-mycorrhizal root length in cm (see example in table below).
- Giovannetti & Mosse (1980) recommend that at a minimum 100 intersections should be used to assess a sample, and found that accuracy was improved if samples were re-randomized and counted several times.
- A much simpler procedure, where an observer simply provides a visual estimate of the degree of mycorrhizal colonization (within 5 or 10 %) can also be reliable (Giovannetti & Mosse 1980). While this method is subjective and prone to operator bias, it still can provide sufficient information when precise values are not required (for pot culture quality control, or when looking at samples from the field).
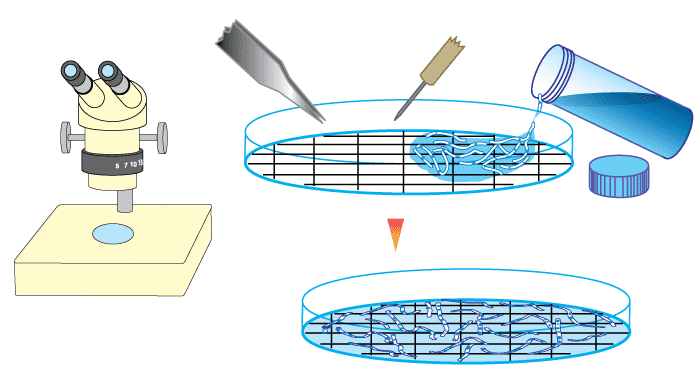 |
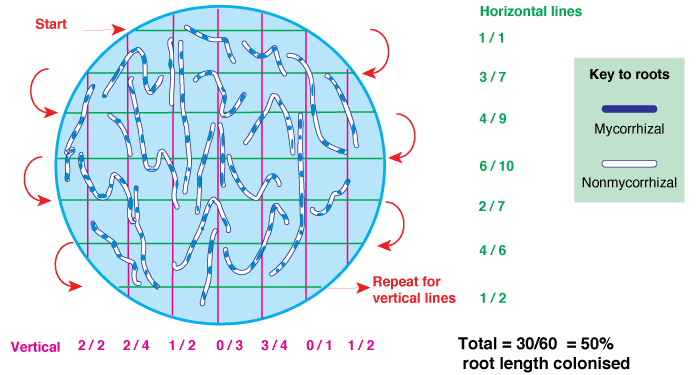 |
A gridline intersection example using a 8.5 cm diameter round Petri dish with a 1/2 inch (14/11 cm) grid, and a 1 m test sample of thread cut into fragments and randomly re-distributed 10 times (Figure 4.3 in Brundrett et al. 1996). Row and column totals are summarised in the table below. |
| Re-distribution | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Intersects (cm) | 102 | 107 | 91 | 98 | 92 | 114 | 108 | 99 | 104 | 94 |
| Average | 100.9 cm ± 2.5 (standard error) | |||||||||
- When mycorrhizal colonization is being assessed using a dissecting microscope, it is always a good idea to make slides from a sub-sample of roots for observation with a compound microscope. This will allow fungi that do not stain well (such as many Acaulospora and some Glomus species) to be seen, and the contribution of saprobic or parasitic fungi to be determined. The contribution of different morphotypes of mycorrhizal fungi can also be estimated.
- A compound microscope with an eye piece cross-hair moved to randomly selected positions can be used to measure the length of arbuscules, vesicles and internal hyphae within roots (McGonigle et al. 1990), as shown below.
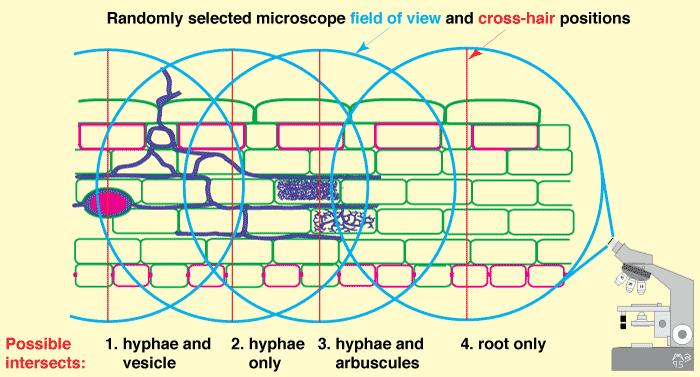 |
Microscopic examination of roots to quantify arbuscular mycorrhizas (Figure 4.4B in Brundrett et al. 1996). |
2. Quantifying Ectomycorrhizal Associations
- A variety of methods have been used to quantify ECM roots. Unstained ECM roots can usually be distinguished from non-mycorrhizal roots by differences in their colour, thickness, texture and branching patterns. However, a clearing and staining or sectioning procedure (see above) is necessary to visualise the Hartig net to confirm that an ectomycorrhizal association is present. A post-clearing bleaching step to remove excess tannins, often helps reveal the Hartig net in ECM roots.
- ECM roots are usually quantified by sampling seedlings, or washing roots from soil cores, taking care to exclude contaminating roots of non-target species. Assuming that roots are young and healthy, each mycorrhizal root tip will contain an active Hartig net zone (where active exchange processes are thought to occur). These tips can be counted to quantify the intensity of the association and their numbers should be expressed relative to root length and soil volume.
- The root length of a sample can be measured with the gridline intersect method, while either simultaneously measuring the length of mycorrhizal roots, or separately counting the total number of mycorrhizal tips (see below).
- Ectomycorrhizal association of may be difficult to recognise in ustained roots if there are minimal changes to branching and thickness the host root.
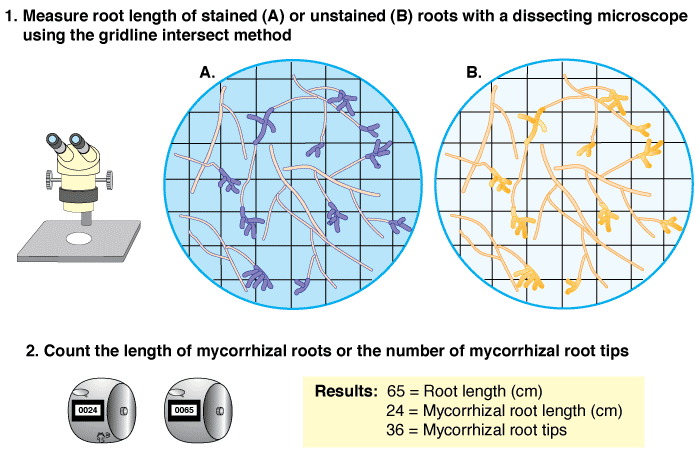 |
Using the gridline intersection method to quantify ectomycorrhizal roots (Figure 4.5A in Brundrett et al. 1996). |
E. Designating Mycorrhizal and Nonmycorrhizal Roots
Different criteria have been used to designate plants with mycorrhizal associations and this has sometimes lead to confusion in the mycorrhizal literature (Harley & Smith 1983, Brundrett 1991). A comprehensive definition of mycorrhizas is presented in Section 1, but definitions of nonmycorrhizal (NM) roots and different categories of mycorrhizas are also required to consistently identify mycorrhizas. Consequently the correct identification of mycorrhizas requires evidence based on all of these definitions. Definition used to recognise VAM and ECM associations are discussed below using protocols from Brundrett et al. 1996 (Section 1.6 p. 32-37).
1. Arbuscular Mycorrhizal (VAM) Associations
The presence of arbuscules in a root are used to designate plants with VAM. However, these structures are ephemeral and may be absent from field-collected roots. Consequently, hyphal colonization alone is often used to identify VAM associations, but hyphae and vesicles of VAM fungi will also occupy non-host roots (see below). Hyphal coils or longitudinal hyphae, but not arbuscules, may be seen in roots collected form the field for the following reasons.
- Roots of many species persist in the soil for months or years without secondary growth, but arbuscules are ephemeral structures that last for only a few weeks (Brundrett & Kendrick 1990). However, it should always be possible to find arbuscules if samples contain young roots (with growing tips).
- Roots from the field are often heavily pigmented with phenolics and other secondary metabolites and may require a post-clearing bleaching step.
- Arbuscules may be harder to see in plants with coiling VAM, or or incases where they occur in a single cortex layer (Brundrett & Kendrick 1990).
- In ecosystem studies, saprophytic colonization of non-host roots, rhizome scales, etc. by hyphae and vesicles of Glomeromycotan fungi is relatively common, as shown in the case study below.
- Exploitative VAM associations of myco-heterotrophic plants lack arbuscules.
Consequently, determining if older roots had VAM may require prior knowledge of that plant species. This knowledge includes root phenology information and prior observations of the same or closely related species. More information will result in safer conclusions.
2. Ectomycorrhizal (ECM) Associations
The presence of a Hartig net, consisting of labyrinthine hyphae between root epidermal or cortex cells, is normally used to identify ECM roots (Harley & Smith 1983). Mycorrhizal experiments have shown that Hartig net formation is a good indicator of host-fungus compatibility and is correlated with host growth responses (Tonkin et al. 1989, Burgess et al. 1994, Dell et al. 1994). Morphological definitions are used to identify mycorrhizal associations. Ectendomycorrhizas, arbutoid and monotropoid associations are all considered to be types of ECM associations (Section 1). Some plants have both ECM and VAM, as explained in the section on dual associations.
Observations of the fruiting of putative ECM fungi near a potential host plant does not provide sufficient evidence to confirm the presence of an association (Harley & Smith 1983). Problems arise if fungi fruit a considerable distance from their host tree or are wrongly assumed to form ECM associations. One such example, explained below, involves the fungus Gyrodon (Boletinellus) merulioides which was thought for many years to be an ECM associate of ash trees (Fraxinus americana) – a tree which only has VAM. It is now known this fungus forms an association with aphids which are parasitic on Fraxinus roots (Brundrett & Kendrick 1987).
3. Facultative Associations and Nonmycorrhizal Plants
Detailed examinations of plants in natural ecosystems often show consistent differences between host plants in both the intensity and consistency of mycorrhiza formation (proportion of root system involved). These observations have shown that species generally either have consistently high levels of mycorrhizas, low, or variable levels of mycorrhizas, or are not mycorrhizal (Janos 1980, Brundrett & Kendrick 1988). Plants belonging to these categories are designated as obligatorily mycorrhizal, facultatively mycorrhizal, or nonmycorrhizal as explained in Section 7. Plants with facultative mycorrhizas consistently have low levels of colonisation (i.e. under 25 % of root length) (Janos 1980, Brundrett & Kendrick 1988). Characteristics of plant roots systems, especially root hair length and abundance, are usually correlated with the degree of mycorrhizal formation, as summarized in the table below.
Typical features of host root systems and mycorrhizal formation that are associated with categories of mycorrhizal formation.
| Designation: | Obligate | Facultative | Nonmycorrhizal |
| Colonization: | |||
| Arbuscules | young roots | sparse or variable | none |
| hyphae or vesicles | older roots | sparse or variable | may occur in old roots |
| Roots: | |||
| diameter | often coarse | usually fine | usually fine |
| root hairs | few/short | many/long | many/long |
4. Endophytic Associations
Endophytic fungi with symptomless associations are common in the roots of plants (Addy et al. 2005. Schultz & Boyle 2005, Schultz et al. 2006). Endophytic growth by mycorrhizal fungi is fairly common and differs primarily from mycorrhizal associations formed by the same fungi elsewhere by the lack of arbuscules or coordinated development in young roots (Brundrett 2004, 2006). Saprophytic activity also differs from mycorrhizal associations, because the roots involved usually are senescent, often also contain saprophytic fungi. Endophytic colonisation of NM plants is considered to be of limited functional significance because does not result in plant growth responses (Ocampo 1986, Muthukumar et al. 1997, Giovannetti & Sbrana 1998).
Glomeromycotan fungi are ubiquitous soil organisms that can colonize a variety of substrates, including rhizome scales and senescing roots of NM and ECM species (St. John et al. 1983, Harley & Harley 1987, Brundrett & Kendrick 1988, Cázares & Trappe 1993, Smith et al. 1998, Imhof 2001). Endophytic activity is distinguished from functional dual ECM/VAM associations by the absence of arbuscules. It is probable that some reports of VAM in plants from predominantly NM families result from saprophytic activity, if arbuscules were not used to definite VAM. It is less likely that these reports result from misidentification of the fungus, since hyphae and vesicles have a characteristic appearance.
Non-symbiotic fungi also often grow on the surface of roots and sometimes this resembles ECM associations, but these roots lack a Hartig net and the morphological responses (swelling and branching of short roots) found in true ECM. Examples of fungal growth on Acer and Scaevola roots are illustrated below and discussed in Section 8. Fungi colonizing the root surface may be beneficial, harmful or neutral to plants.
Examples of endophytic growth by mycorrhizal fungi and other fungi are illustrated in the case studies provided below.
5. Recommendations
The presence of arbuscules should be used to identify VAM and the presence of a Hartig net to define ECM associations. However, these definitions are not always applied and careful judgment may be required when interpreting mycorrhizal associations in roots collected from the field, particularly if roots are old, or associations are atypical.
There is disagreement about whether arbuscular mycorrhizas or vesicular-arbuscular mycorrhizas is the most appropriate name for these associations (see Brundrett 2004). The name arbuscular mycorrhizas has gradually become more common than vesicular-arbuscular mycorrhizas in the scientific literature. It is advisable to use both the words arbuscule and Glomeromycotan in the title or keywords of papers to ensure they will be retrieved by computerised search programs in the future.
The terms ectomycorrhiza / ectomycorrhizas / ectomycorrhizal should be used for ECM associations.
The degree of mycorrhizal formation of a host plant is usually expressed as the proportion (%) of root length colonized by mycorrhizal fungi. However, roots with a periderm (bark) layer resulting from secondary growth should be excluded, since they have no cortex. In ecosystem surveys, it is best to express the degree of mycorrhizal colonization as the proportion of roots available for colonization that were mycorrhizal. This requires an understanding of the dynamics of root growth and mycorrhizal formation.
F. Case Studies
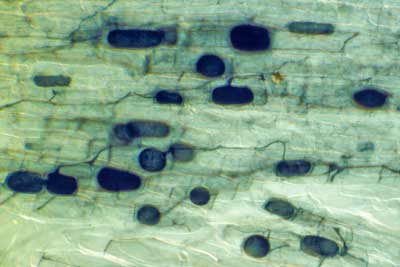 |
Hyphae (arrows) and vesicles (V) of a Glomeromycotan fungus within a rhizome scale of Hydrophyllum virginianum, a nonmycorrhizal plant. This is saprophytic activity without arbuscules (Brundrett & Kendrick 1988).
|
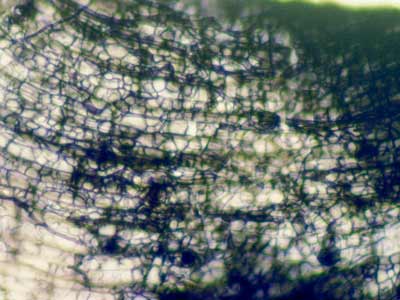 |
Hyphal growth on the surface of roots is sometimes mistaken for ECM. This unidentified fungus is growing on roots of Acer saccharum (Sugar Maple) a VAM host from Canada.
|
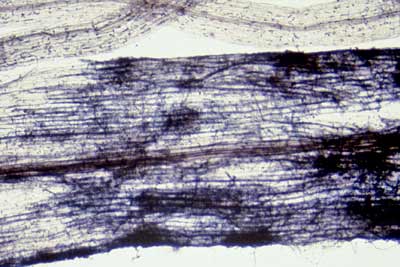 |
An unidentified fungus growing on roots of Scaevola calliptera (Fan Flower) a VAM host from Western Australia. Members of this family (Goodeniaceae) were reported to have ECM-like associations without a Hartig net (Warcup 1980).
|
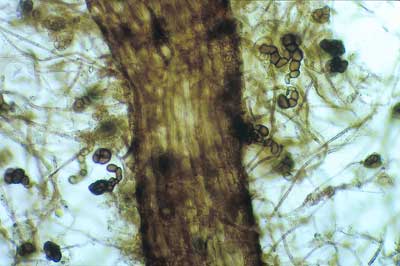 |
Unusual associations can occur in nursery-grown tree seedlings in the absence of more typical ECM fungi. In this case a conidial fungus has formed “superficial” ECM in nursery grown Eucalyptus seedlings with a thin mantle.
|
 |
The ash bolete (Gyrodon merulioides) specifically fruits under ash trees (Fraxinus americana) in North America, so was listed as an ECM fungus, even though ash trees only have VAM. However, G. merulioides actually has a mutualistic association with root-feeding aphids it protects within hollow black sclerotia (Brundrett & Kendrick 1988).
|
Version 2.0 © Mark Brundrett 2008