MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 9. ECTOMYCORRHIZAL FUNGI
A. Introduction
This section provides a brief introduction to ectomycorrhizal fungus diversity, primarily using examples from Australia. More information is available using the links provided below.
Images on this page are by Neale Bougher (Western Australian Department of Environment and Conservation) and Mark Brundrett, unless otherwise stated and cannot be used without permission. Many of Neale Bougher's photos are from the Perth Urban Bushland Fungi Field Book, which contains more information about these species.
Sources of information on Australian Fungi
- Catalogue of Australian Fungi
- Catalogue of New Zealand Fungi
- Fungimap
- Perth Urban Bushland Fungi Project
- Australasian Mycological Society
- Australian National Botanic Gardens Fungi website
- Fungibank
- Sydney Fungal Studies Group
B. Structural Diversity
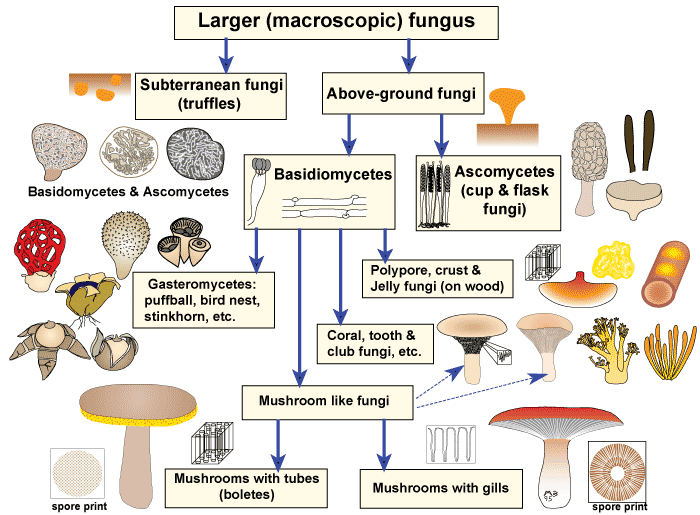
Most fungi have fruit-bodies that are clearly visible for a brief period usually in the autumn in temperate regions, or during the wet season in tropical regions, when spores are dispersed. As illustrated in the diagram below, there is a wide taxonomic and structural diversity of visible fungi (called macrofungi or larger fungi). The reproductive structures of larger fungi include epigeous mushrooms, puffballs, coral fungi, crust fungi, etc. and subterranean (hypogeal) fungi called truffles, or truffle-like fungi. Most of these categories contain ectomycorrhizal fungi.
Hover mouse over diagram to see which categories contain ectomycorrhizal fungi (ECMF)
C. Preliminary list of Ectomycorrhizal Fungi
The majority of ECM fungi are Basidiomycetes, with some Ascomycetes and a few Zygomycetes, as listed in the Table below. Fungi in this table are organised into clades based on molecular phylogeny with genera confirmed to be symbiotic plant associates indicated. The arrangement of clades in the fungal tree of life is not fully resolved.
| Clade No. | Family or Group | Epigeous Larger Fungi (mushrooms, puffballs, etc.) | Truffles (hypogeous, or semi-hypogeous) | Resupinate (crust fungi) |
| Basidiomycetes | ||||
| 1 (34) | Lyophyllum | Lyophyllum* | ||
| 2 (37-38) | Tricholomataceae | Leucopaxillus, Tricholoma* | ||
| 3 (43/44) | Entolomataceae | Entoloma* (not all species) | Rhodogaster, Richoniella | |
| 4 (55) | Amanitaceae | Amanita Limacella | Ammarrendia, Torrendia | |
| 5 (65) | Hygrophoraceae | Gliophorus, Humidicutis, Hygrophorus* | ||
| 6 (72) | Hydnangiaceae (Laccaria clade) | Laccaria | Hydnangium*, Podohydnangium, Gigaspera | |
| 7 (73) | Cortinariaceae | Cortinarius*, Dermocybe, Naucoria*, Rozites* | Cortinarius, Hymenogaster (in part), Destuntzia, Protoglossum, Quadrispora, Setchelliogaster*, Stephanopus*, Thaxterogaster* | |
| 8 (74) | Phaeocollybia clade in Hymenogastraceae |
Phaeocollybia* | ||
| 9 (95) | Hebeloma clade in Hymenogastraceae |
Hebeloma* | Hymenogaster (in part) | |
| 10 (78/79) | Descolea clade (in Bolbitiaceae?) |
Descolea* | Descomyces*, Setchelliogaster | |
| 11 (104) | Inocybaceae | Inocybe*, Auritella | Auritella | |
| 12A (Outgroup 1) |
Boletes (Boletales) Includes many families | Aureoboletus, Austroboletus, Austropaxillus*, Boletellus*, Boletochaete, Boletus*, Chroogomphus*, Fuscoboletinus, Heimielia, Gomphidius*, Gyroporus*, Leccinum*, Paragyrodon, Paxillus*, Phlebopus, Phylloporus, Poryphyrellus*, Psiloboletinus, Rubinoboletus, Strobilomyces, Suillus*, Tylopilus*, Xanthoconium, Xerocomus*, etc. | Alpova*, Austrogaster, Austrogautieria, Chamonixia*, Gastroboletus, Gastrotylopilus, Gymnogaster, Gymnopaxillus, Horakiella, Hysterogaster, Melanogaster*, Mycoamaranthus, Octaviania, Rhizopogon*, Royoungia, Sclerogaster, Truncocolumella*, Timgrovea, Wakefieldia, etc. | (Serpula, etc. are saprophytes) |
| 12B (Outgroup 1) |
Boletales - Sclerodermatales | Boletoid: Gyroporus, Gasteroid: Pisolithus*, Scleroderma*, Calostoma* |
Astaeus*, Horakiella, Scleroderma,, Velligaster | |
| 13 (Outgroup 2) |
Russulales | Lactarius*, Russula* | Arcangeliella*, Cystangium, Gymnomyces, Leucogaster, Macowanites, Octaviania*, Stephanospora, Zelleromyces | Byssoporia, Albatrellus*, Polyporoletus* (Stereum, etc. are saprophytes) |
| 14 (Outgroup 3) |
Gomphales & Hysterangiales | Bankera*, Boletopsis*, Clavaridelphus*, Gomphus*, Hydnum*, Hydnellum*, Phellodon*, Sarcodon*, Ramaria* (some NM?) | Hysterangium clades: Aroramyces, Chondrogaster, Hysterangium* , Mesophelliaceae: Andebbia, Castoreum, Gummiglobus, Malajczukia, Mesophellia, Nothocastoreum, Gallacaceaceae: Austrogautiera, Gallacea, etc., Phallogaster clade: Protrubera, Phallogaster, Trappea Ramaria clade: Gautieria* |
|
| 15 (Outgroup 4) |
Cantharellales | Cantharellula, Cantharellus*, Craterellus* | Sistotrema? | |
| 16AB (Outgroup(s) 5) |
Rhizoctonia alliance clades (Sebacinaceae, Ceratobasidiaceae) | Thanatephorus*, Sebacina* | ||
| 17 (Outgroup 6) |
Thelephorales | Bankera, Boletopsis, Thelephora*, Phellodon*, Sarcodon | Tomentella*, Pseudotomentella*, Tomentellopsis* | |
| 18 (Outgroup 7) |
Clavariaceae | Aphelaria, Clavaria, Clavariadelphus, Clavicorona, Clavulina, Clavulinopsis, Ramariopsis | ||
| 19 (Outgroup 8) |
Atheliales | Amphinema*, Byssocorticium*, Byssosporia*, Piloderma*, Tylospora* | ||
| Ascomycetes | ||||
| 20 (Ascomycete-1) | Pezizales (inc. Tuberaceae, Helvellaceae, etc.) | Genea*, Geopora, Humaria*, Hydnotrya, Helvella, Leucangium*, Pachyphloeus, Pulvinula, Sarcosphaera, Sphaerosporella*, Sphaerozone*, Tirmania, Tricharina*, Wilcoxina* | Choiromyces, Dingleya, Eremiomyces, Kalaharituber, Labyrinthomyces, Pachyphloeus, Reddellomyces, Tuber*, Terfezia, Turmania | Phialophora* (anamorph) |
| 21 (Ascomycete-2) | Eurotiales (Elaphomycetaceae) | Elaphomyces* Pseudotulostoma* | ||
| 22 (Ascomycete-3) | Melanommatales | Cenococcum (sterile fungus) | ||
| Zygomycetes | ||||
| 23 (Zygomycete-1) | Endogonaceae | Densospora, Endogone, Peridiospora, Youngiomyces | ||
- Clades
- Fungal clades are provided in the first column are ordered using the 117 clade classification of the euagarics by Moncalvo et al. 2002, with outgroups numbered to reflect increasing distance from the agarics in that tree.
- Orders and Families
- Follow Matheny et al. 2006 and Hibbett et al. 2007.
- Genera
- Follow cited references (some are synonyms).
- Classification and Identification References
- Arora 1986, 1991, LoBuglio et al. 1996, Binder et al. 1997, Erland & Taylor 1999, Kõljag et al. 2000, Bougher & Lebel 2001, Humpert et al. 2001, Peinter et al. 2001, Moncalvo et al. 2000, 2002, Binder & Bresinsky 2002, Selosse et al. 2002, Tehler et al 2003, Urban et al. 2003, Binder et al. 2005, Douhan & Rizzo 2005, Ferdman et al. 2005, Henkel et al. 2006, Matheny & Bougher 2006, Matheny et al. 2006, Tedersoo et al. 2006, Trocha et al. 2006, Watling 2006, Barroetaveña et al. 2007, Hibbett et al. 2007, Hosaka et al. 2007, Wilson et al. 2007.
- Genera in Bold
- Confirmed ECM Associations by fungal isolation and re-synthesis under controlled conditions: Warcup 1980, 1985, Kropp & Trappe 1982, Malacjzuk et al. 1982, Molina & Trappe 1982a, Bougher et al. 1985, Godbout & Fortin 1985, Reddell & Milnes 1992, Burgess et al. 1993, Massicotte et al. 94, Thomson et al. 1994, Cripps & Miller 1995, McGee 1996, Kawai 1997, Lu et al. 1998, Reddell et al. 1999, Baxter & Dighton 2001, Yamada et al. 2001ab, Yun & Hall 2004, Brundrett et al. 2005, Henkel et al. 2006.
- Genera with an Asterisk(*)
- Links between fruitbodies and ECM morphotypes established, as summarised by Agerer 2006. Additional information: Norvell 1998, Wilson et al. 2007.
- Excluded Taxa
- These include:
(i) Catathelasma seems to be ectomycorrhizal, but this is not fully resolved (Hutchison 1992).
(ii) Truffle-like fungi that may be saprobic: Endoptychum, Cribbea, Protubera, Gelopellis, Secotium, Stephanospora, Gastrocybe, Weraroa, Tympanella, etc.
(iii) Ascomycetes that fruit after fire (discomycetes), such as Peziza, Morchella, and Scutellinia, considered to be saprobes (see Fujimura et al. 2005).
(iv) The bolete - Gyrodon (Boletinellus) merulioides is not mycorrhizal (see Section 10).
D. Examples of Ectomycorrhizal Fungi
Examples of larger genera of ECM fungi are provided below, primarily using fungi from Australia and New Zealand. Most of the major groups of ECM fungi are well represented in Australia, including over 700 species that have been named (Bougher 1995, Bougher & Lebel 2001). The majority of these fungi are endemic. However, taxonomic studies on Australian fungi are at an early stage compared to the Northern Hemisphere and new species are continually being discovered (Pascoe 1991, May 2001, Bougher & Lebel 2001). Information on Australian fungi can be obtained from books and the websites listed above.
Laboratory and glasshouse experiments have shown that different fungi vary in their capacity to utilise resources, withstand adverse conditions, etc. For this reason, mycorrhizal fungus diversity is thought to contribute to the resilience of ecosystems, but there is very little comparative data that can be used to test this hypothesis.
| 2. Tricholomataceae | |

|
 |
Fungus: Tricholoma sp.
|
Fungus: Tricholoma sp. (Matsutake)
|
| 3. Entolomataceae | |
 |
Fungus: Entoloma moongum
|
| 8. Hebeloma a Clade in the Hymenogastraceae | |
 |
Fungus: Hebeloma westraliense
|
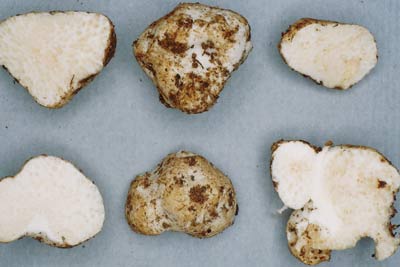
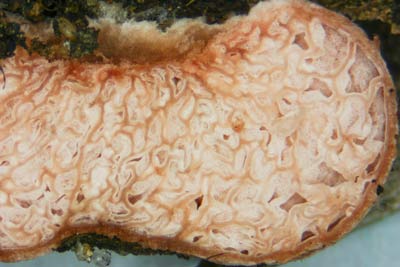
| 10. Descolea a Clade in the Bolbitiaceae | |
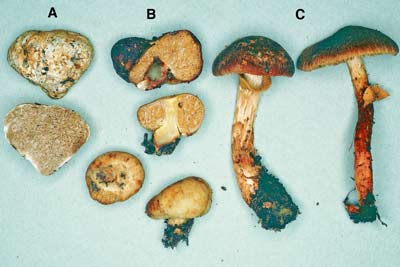 |
Fungus: Descolea (C), with its truffle-like relatives Setchelliogaster (B) and Descomyces (A)
|
| 11. Inocybaceae | |
 |
Fungus: Inocybe violaceocaulis
|
| 14. Gomphales | |
 |
 |
Fungus: Ramaria ochraceosalmonicolor
|
Fungus: Ramaria abitina
|
 |
 |
Fungus: Ramaria versatilis
|
Fungus: Gomphus floccosus |
| 15. Cantarelloid clade | |
 |
 |
Fungus: Cantharellus sp.
|
Fungus: Craterellus cornicopioides Horn of Plenty
|
 |
Fungus: Tomentella sp.
|
Mycorrhiza: associated with this crust fungus  |
| 18. Clavariaceae | |
 |
Fungus: Clavaria amoena
|
| 20. Pezizales (Ascomycetes) | |
 |
Fungus: Labyrinthomyces sp.
|
 |
Fungus: Hydnoplicata convoluta
|

| Fungus: Tuber melanospora
|
| 22. Melanommatales (Ascomycetes) | |
 |
Fungus: Cenococcum sp.
|
| 23. Endogonaceae (Zygomycetes) | |
 |
Fungus: Endogone pisiformis
|
E. Ectomycorrhizal Morphotypes
Characteristic features of ECM root tips used to identify associated fungi (Agerer 1986, Ingleby et al. 1990, Haug & Pritsch 1992) are briefly summarised in a table below. Some examples of roots colonised by known fungi in glasshouse or sterile culture experiments are also provided. Comprehensive information on mycorrhizal morphotypes is provided on the Deemy website.
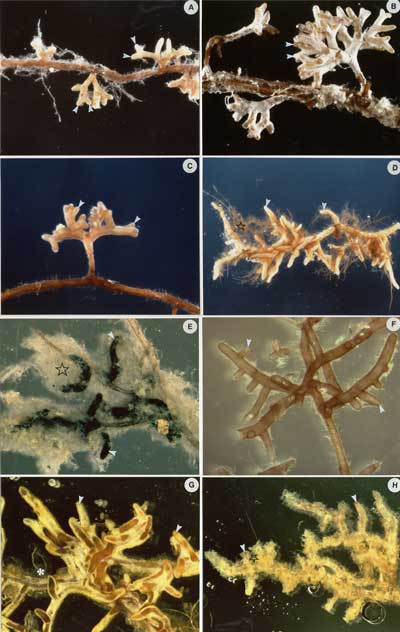 |
| ||||||||||
 |
 |
Synthesised ECM associations of Thanatephorus gardneri (Rhizoctonia alliance) and Melaleuca sp. grown in the glasshouse. Note relatively thin reddish ECM roots and thick black mycelia. |
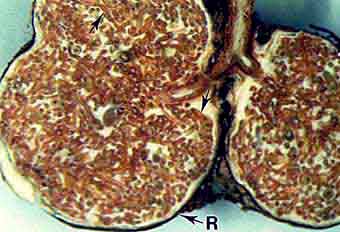
Tuberculate ECM of Eucalyptus pilularis collected in Queensland. Numerous ECM roots (arrows) are encased within an outer rind (R). R = peridium, |
Table of diagnostic features of ectomycorrhizal roots that may be associated with particular ectomycorrhizal fungi.
Mycorrhizal development
- root branching patterns and density
- root thickening
- reduction in root elongation
External hyphae
- presence, abundance, distribution
- arrangement - single, strands, rhizomorphs
- structure - colour, wall, thickness, clamp connections, crystals
- sclerotia
Mantle
- thickness
- colour, changes with bruising, or age
- hyphal organization in surface and in deeper layers
- loose or compact hyphae or pseudo-parenchyma, prosenchyma
- hyphae - thickness, wall structure, clamp connections, septae
- cystidia, crystals, exudates
- reactions to chemicals (Melzer's reagent, KOH, etc.)
- staining reactions
- odour
- fluorescence
Hartig net
- presence, thickness
- hyphal organisation
- hyphal structure
Version 2 © Mark Brundrett 2008
Images © Mark Brundrett & Neale Bougher