MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 7. ROLES OF MYCORRHIZAL ASSOCIATIONS
A. Introduction
This Section briefly describes how mycorrhizal associations help plants grow and the roles mycorrhizal fungi in ecosystems. Text books on mycorrhizal associations and plant nutrition that can be consulted for more information are listed in the table below. The principal sources of information cited below is the book: Working with Mycorrhizas in Forestry and Agriculture (Brundrett et al. 1996) and a review article on mycorrhizal ecology (Brundrett 1991).
Further Information
| Topic | Source |
| Plant Nutrition | Marschner 1995, Lambers et al. 2008 |
| Mycorrhizal physiology | Smith & Read 1997 |
| Mycorrhizal Ecology | Allen 1991, Brundrett 1991 |
B. Why Mycorrhizas are Important
Suggested roles of mycorrhizal fungi in natural and managed ecosystems are listed below (after Brundrett & Abbott 2002, Brundrett & Cairney 2002).
A. Benefits to plants
- Increased plant nutrient supply by extending the volume of soil accessible to plants as explained below.
- Increased plant nutrient supply by acquiring nutrient forms that would not normally be available to plants (Tarafdar & Marschner 1994, Schweiger et al. 1995, Kahiluoto & Vestberg 1998).
- Some ECM and ericoid fungi have the capacity to breakdown phenolic compounds in soils which can interfere with nutrient uptake (Bending & Read 1997).
- Root colonisation by ECM and VAM fungi can provide protection from parasitic fungi and nematodes (Duchesne et al. 1989, Grandmaison et al. 1993, Newsham et al. 1995, Little & Maun 1996, Cordier et al. 1998, Morin et al. 1999).
- Non-nutritional benefits to plants due to changes in water relations, phytohormone levels, carbon assimilation, etc. have been reported, but are difficult to interpret (Brundrett 1991, Smith & Read 1997).
- Mycorrhizal benefits can include greater yield, nutrient accumulation, and/or reproductive success (Lewis & Koide 1990, Stanley et al. 1993).
- Mycorrhizas can cause growth form changes to root architecture, vascular tissue, etc. (Daniels Hetrick et al. 1988, Miller et al. 1997).
- Suppression of competing non-host plants, by mycorrhizal fungi has been observed (Allen et al. 1989).
- Significant amounts of carbon transfer through fungus mycelia connecting different plant species has been measured (Simard et al. 1997). This could reduce competition between plants and contribute to the stability and diversity of ecosystems.
- Networks of hyphae supported by dominant trees may help seedlings become established or contribute to the growth of shaded understorey plants (Hogberg et al. 1999, Horton et al. 1999).
- Nutrient transfer from dead to living plants may occur (Eason et al. 1991).
B. Other roles in ecosystems
- Soil hyphae are likely to have an important role in nutrient cycling by helping to prevent losses from the system, especially at times when roots are inactive (Lussenhop & Fogel 1999).
- Hyphae are conduits that may transport carbon from plant roots to other soil organisms involved in nutrient cycling processes. Thus, cooperating with other members of the decomposition soil food-web.
- Soil hyphae may have an important role in nutrient cycling by acquiring nutrients from saprophytic fungi (Lindahl et al. 1999).
- Epigeous and hypogeous sporocarps of ECM and VAM fungi are important food sources for placental and marsupial mammals (McGee & Baczocha 1994, Janos et al. 1995, Reddell et al. 1997, Mcilwee & Johnson 1998, Claridge 2002).
- Mycorrhizal roots and fungus fruit bodies are important as food sources and habitats for invertebrates (Fogel & Peck 1975, Rabatin & Stinner 1989, Lawrence & Milner 1996).
- Mycorrhizal fungus hyphae are an important food source for soil invertibrates (Setala 1995, Ingham & Massicotte 1994).
- Mycorrhizas influence soil microbial populations and exudates in the mycorrhizosphere and hyphosphere (Ames et al. 1984, Bansal & Mukerji 1994, Olsson et al. 1996, Andrade et al. 1998).
- Hyphae of VAM fungi are considered to contribute to soil structure. Their role in mechanical aggregation has been questioned (Degens et al. 1994), but secretions such as glomalin may be more important (Wright & Upadhyaya 1998). Hyphal mats produced by ECM fungi considerably alter soil structure (Griffiths et al. 1994).
- Mycorrhizal fungi contribute to carbon storage in soil by altering the quality and quantity of soil organic matter (Ryglewicz & Andersen 1994).
C. Values to people
- ECM fungi are economically and nutritionally important as human food resources (Arora 1991, Kalotas 1996).
- These mushrooms have also have been used as medicines and natural dyes (Arora 1991, Morgan 1995).
- Larger fungi have aesthetic values and are an important part of the as culture, folklore and appreciation of nature by many people (Findlay 1982, Morgan 1995).
- Mycorrhizas can influence the nutritional quality of food by influencing uptake of micronutrients and pollutants (Joner & Leyval 1997).
- Fungal diversity is a bio-indicator of environmental quality.
- Fungi which have adapted to local soil conditions are required for agriculture, horticulture and forestry.
C. How Mycorrhizas Work
Mycorrhizal associations are defined in the introduction section. Mineral nutrient acquisition from soil is considered to be the primary function of mycorrhizas, but other roles for these fungi have been suggested, as listed in B above. Only the uptake of nutrients is discussed here.
1. Nutrient Depletion Zones
The demand for a particular mineral nutrient depends on plant internal requirements, while the supply of that nutrient primarily depends on its availability and mobility in soils (Russell 1977, Marschner 1995).
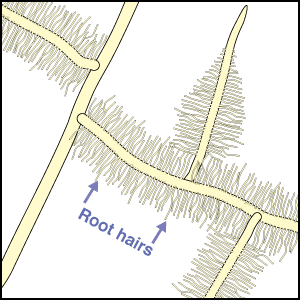
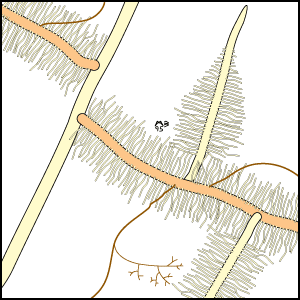
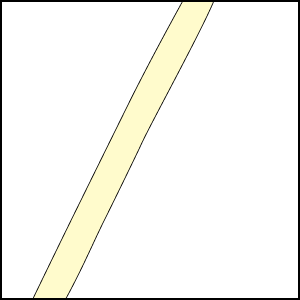
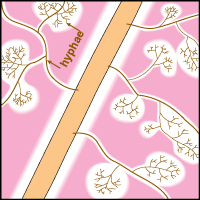
Mineral nutrients such as phosphorus have very limited mobility in soils so that depletion zones - where all the available nutrient has been utilised, quickly from around roots (Bhat & Nye 1974, Russell 1977, Marschner 1995). Thus to obtain more phosphorus, plants must bypass these depletion zones by further root activity elsewhere in the soil. The outcome of this quest for phosphorus (and other relatively immobile soil resources) should largely be determined by the surface area of a plant's root system. The most important role of mycorrhizal fungus hyphae is to extend the surface area of roots as is explained in diagrammatic form below. The capacity of plants to influence nutrient availability in soils will also depend on the extensiveness and activity of their root system, since young roots are the primary source of exudates (Curl & Truelove 1980, Uren & Reisenaur 1988).
| MYCORRHIZAS ABSENT | MYCORRHIZAS PRESENT | |
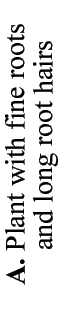 |
 |
 |
| Substantial mycorrhizal benefits unlikely in this case | ||
 |
 |
 |
| Substantial mycorrhizal benefits are likely with this type of root | ||
| Caption |  |
 |
- Instructions
- Hover mouse over drawings to see Phoshorus (P) depletion zone
- Notes
- These diagrams have been simplified to assume that phosphorus is uniformly distributed in the soil and is equally available to roots and hyphae. They show the extreme situations of a plant with very fine roots and long hairs (such as many grasses) and a plant with thick roots and no root hairs. There are also many plants with intermediate root systems.
- Static Images
- A static image is also provided to download.
| Nonmycorrhizal | Mycorrhizal | P Depletion Zone |
 |
 |
|
 |
 |
|
2. Hyphal Activity
Mycorrhizal fungus hyphae are considered to function primarily by increasing the soil volume from which available forms of phosphorus are absorbed and provided to roots. Hyphae of VAM fungi can respond to localised sources of soil nutrients more rapidly than roots (St John et al. 1983, Warner 1984) and to produce fine highly-branched "absorptive" hyphae in decomposing organic substrates (Mosse 1959, Nicholson 1959, Bago et al. 1998). ECM fungus hyphae also proliferation in decomposing soil organic matter (Harvey et al. 1976, Reddell & Malajczuk 1984, Bending & Read 1995).
Hyphae of VAM fungi can utilise the same forms of nutrients as roots, but these associations seem to have a greater benefit when phosphorus is present in less-soluble forms (Bolan 1991, Schweiger 1994, Tarafdar & Marschner 1994, Kahiluoto & Vestberg 1998). Hyphae of ECM fungi utilise both inorganic and simple organic sources of nitrogen and phosphorus (Abuzinadah & Read 1989, Hausling & Marschner 1989). Some ECM fungi occur is soils enriched with ammonium (Sagara 1992). Soil mineralisation by organic acids produced by hyphae has been measured within ECM fungus mats (Griffiths & Caldwell 1992). ECM fungus hyphae can also grow within rocks where they contribute to mineral weathering (Jongmans et al. 1997, van Breemen et al. 2000). Thus, mycorrhizal fungi may have access to forms of nutrients which are not directly available to plants, in addition to acquiring nutrients which are spatially or chronologically separated from roots.
| VAM | ECM |
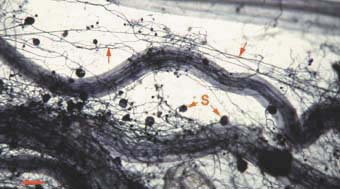 |
 |
D. Mycorrhizal Dependency
The concept that plants have varying degrees of dependence on mycorrhizal associations is gaining acceptance (Janos 1980, St John 1980, Marschner 1986, Brundrett 1991). Detailed examinations of plants in natural ecosystems often show consistent differences between host plant species in the intensity and consistency of mycorrhiza formation (proportion of root system involved). These observations have shown that species generally either have (a) consistently high levels of mycorrhizas, (b) intermediate, or variable levels of mycorrhizas, or (c) are not mycorrhizal (Janos 1980, Brundrett & Kendrick 1988). Plants belonging to these categories can be called obligatorily mycorrhizal, facultatively mycorrhizal, or nonmycorrhizal, as are defined below.
In natural ecosystems, plants with facultatively mycorrhizal associations or nonmycorrhizal (NM) roots are more common in very dry, wet or cold habitats where plant productivity is limited by soil/environmental conditions, or in disturbed habitats where mycorrhizal fungus inoculum is limited (Brundrett 1991). Nonmycorrhizal trees are rare (members of the Australian family Proteaceae are one exception). There are a number of NM genera which are important in agriculture and horticulture including members of the families Chenopodiaceae, Amaranthaceae, Caryophyllaceae, Polygonaceae, Brassicaceae, Scrophulariaceae, Commelinaceae, Juncaceae and Cyperaceae (see list provided). Many parasitic and carnivorous plants, and those with cluster or dauciform roots tend to be NM.
1. Definitions
- Mycorrhizal dependency
- A measure of the benefit provided by mycorrhizas and will depend on relative contribution of root and mycorrhizal mediated nutrient uptake to plants (Janos 1980). Mycorrhizal dependency has often been quantified by calculating the yield ratio between mycorrhizal plants and uninoculated control plants grown in a particular soil at a single soil P level (Koide et al. 1988, Manjunath & Habte 1991, Hetrick et al. 1992). However, it is better to analyse mycorrhizal benefits across a range of soil P levels, by producing nutrient response curves like that shown in the Figure below.
- Obligatorily mycorrhizal plants
- Plants which will not survive to reproductive maturity without being associated with mycorrhizal fungi in the soils (or at the fertility levels) of their natural habitats (Janos 1980). These species consistently support mycorrhizal colonization throughout most of their young roots (if inoculum is sufficient).
- Facultatively mycorrhizal plants
- Plants that benefit from mycorrhizal associations only in some of the least fertile soils in which they naturally occur (Janos 1980). In ecosystem surveys, inconsistent mycorrhizas (Trappe 1987) or low levels of mycorrhizal colonization (less than 25 % - Brundrett & Kendrick 1988) have been used to designate facultatively mycorrhizal species when soil fertility levels could not be manipulated.
- Nonmycorrhizal plants
- Plants with roots that consistently resist colonization by mycorrhizal fungi, at least when they are young and healthy (Brundrett 1991). Soil properties, or limited mycorrhizal inoculum can also reduce mycorrhizal formation, but usually do not prevent it completely.
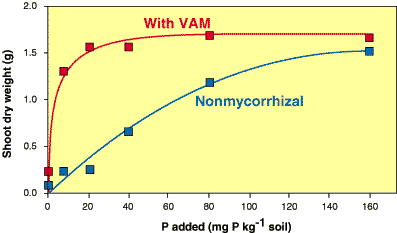 |
This response curve was produced by growing mycorrhizal and nonmycorrhizal plants of the Australian native Cassia pruinosa at a range of soil P levels. Note that the benefit provided by mycorrhizal associations to this species is dependant on soil P levels and can be defined by comparing these two curves (data from Jasper et al. 1994). |
2. Root System Form
The main role of mycorrhizal associations is to acquire nutrients by exploring the soil volume with hyphae that are both more responsive and more extensive than the roots themselves. However, roots of some species of plants are also capable of effectively exploring large soil volumes and responding to temporary soil resources, and these species are likely to have facultative mycorrhizal associations, or be nonmycorrhizal. The production of fine roots or associations with mycorrhizal fungi are two alternative strategies that both require plants to expend metabolic energy on their root systems. It is considered likely that mycorrhizal fungus hyphae should be a more cost effective means of exploring large soil volumes, because of their narrow diameter (Harley 1989). Plants generally do not support both high levels of mycorrhizal colonisation and root systems with fine/active roots, because of the high metabolic cost that would result (Brundrett 1991).
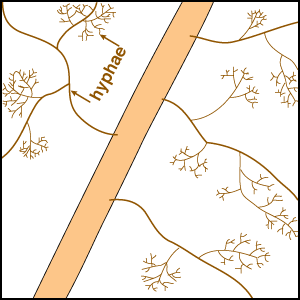
Considerable variations in root system extensiveness, geometry, depth distribution, and plasticity occur between plant species (Grime et al. 1986, Fitter 1987). In experiments, total biomass is the parameter most often used to quantify roots, but provides much less information than root length or specific root length (length/unit root weight) data (Fitter & Hay 1987, Eissenstat 1992). Parameters that could be used to predict nutrient absorption are: total root biomass < fine root biomass < root length < root surface area < root surface area < rhizosphere volume, arranged in increasing order of predictive ability. When root parameters of plant species are compared, root hairs have been found to be most highly correlated with their ability to absorb P from soils (Itoh & Barber 1983, Föhse et al. 1988, Schweiger et al. 1995). Plants with extensive (highly branched, fine, long roots with numerous root hairs) have often been observed to derive little benefit from mycorrhizas in experiments (Bayliss 1975, Koide et al. 1988, Manjunath & Habte 1991, Hetrick et al. 1992).
In addition to have fine roots with long root hairs, there is a tendency for mycorrhizal plants to have roots which are less active (grow slowly, live longer, etc.) than species with little or no mycorrhizal colonisation of their roots (see Table below). The responsiveness, or capacity of plants to exploit small scale or short duration changes in water or nutrient availability by rapidly producing new roots, is thought to be an important determinant of their success during competition for soil resources (St. John et al. 1983, Grime et al. 1986, Fitter 1987). Some plants can also change rhizosphere conditions, such as pH, which may influence nutrient availability (Marschner 1986, Uren & Reisenaur 1988, Lambers et al. 2006). Examples of roots of plants that do not have mycorrhizas are illustrated in Sections 3 and 6.
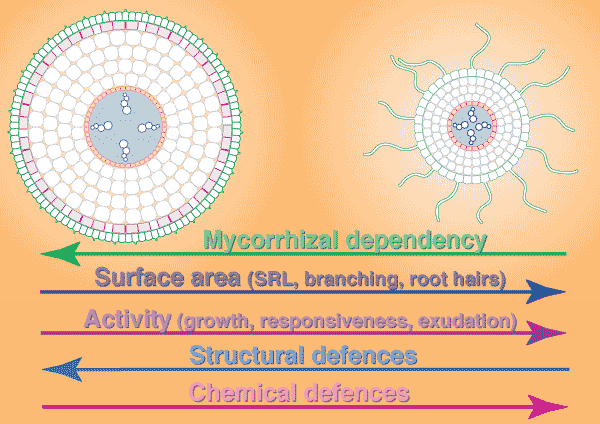 |
Comparison of typical roots of mycorrhizal (left) and nonmycorrhizal (right) plants
|
Host and soil factors which can be used to help predict the potential benefit of mycorrhizas are outlined in the Table below. Assuming that soil nutrient levels are not unusually high and inoculum of appropriate mycorrhizal fungi is available, root strategy differences will primarily determine the magnitude of benefits from mycorrhizal associations.
| GENERALISED RELATIONSHIPS BETWEEN FEATURES OF ROOT SYSTEMS AND THE MYCORRHIZAL DEPENDENCY OF PLANTS | ||
| Root feature |
Mycorrhizal dependency of plant High <----------> Low | |
| A. ROOT SURFACE AREA | low |
high |
| 1. Root system surface area | low | high |
| 2. Branching orders of lateral roots | few | many |
| 3. Branching frequency | sparse | frequent |
| 4. Root hair abundance and length | few/short | many/long |
| B. ROOT ACTIVITY | slow | fast |
| 1. Root growth rate | slow | fast |
| 2. Response to soil conditions | slow | fast |
| 3. Primary root lifespan | long | short |
| 4. Protective structural features | strong | weak |
| 5. Root exudation | ? less | ? more |
| C. MYCORRHIZAL FORMATION | efficient | inefficient
|
Notes: This table is based on differences in root system properties between plants of different mycorrhizal status in Canadian forests (Brundrett & Kendrick 1988, Brundrett 1991). There is only limited data from other ecosystems. The impact of root structures on root exudation is inferred from permeability studies (Ma & Peterson 2003).
E. Soil and Site Factors Influencing Mycorrhizas
Soil factors that can influence the benefits provided by mycorrhizal fungi to plants are considered below.
1. Mycorrhizal Inoculum
Plants with mycorrhizal associations predominate in most natural ecosystems, so inoculum of mycorrhizal fungi is present in most soils. The quantity inoculum of mycorrhizal fungi which are compatible with a host plant in soils can be measured by bioassay experiments. In these experiments, seedlings are grown in intact soil cores or mixed soil samples for sufficient time to allow mycorrhizas to form, then roots are sampled, processed and assessed to measure mycorrhiza formation (Perry et al. 1982, Parke et al. 1984, McAfee & Fortin 1986, Warcup 1991, Brundrett & Abbott 1995).
In Australia, there are fewer species of ECM fungi in sites where eucalypt plantations are established than in undisturbed forests (Lu et al. 1999). There are also cases where plantations of trees have been introduced to habitats where they do not occur naturally. For example, Australian eucalypts are now grown in exotic habitats in Africa, Europe, Asia and South America, habitats which are few compatible ECM fungi, because local fungi are specific to other hosts such as conifers (Malajczuk et al. 1982, Molina et al. 1992).
2. Soil Disturbance
Propagules of mycorrhizal fungi may be absent from soils where severe soil disturbance has resulted in topsoil loss, or where host plants are limited by adverse soil or site factors such as salinity, aridity, waterlogging, or climatic extremes (Brundrett 1991). Most studies of mycorrhizal associations in highly disturbed habitats such as mine sites have found reduced levels of mycorrhizal propagules (Danielson 1985, Jasper et al. 1992, Pfleger et al. 1994, Brundrett et al. 1996). Less severe forms of soil disturbance, including agricultural tillage, soil animal activities, fire and erosion can also reduce levels of mycorrhizal fungus propagules (Habte et al. 1988, O'Halloran et al. 1986, Read & Birch 1988, Vilarino & Arines 1991).
Spores and other propagules of mycorrhizal fungi can introduced to new sites by wind or water erosion, or by the activity of animals which feed on fungi (Allen 1991, Brundrett 1991, Claridge & May 1984, McGee & Baczocha 1994, Janos et al. 1995). In a disturbed habitat, the effectiveness of natural vectors will depend on the proximity of undisturbed habitats containing suitable fungi (and their associated animals) as well as the phenology of fruiting of fungi. Effective colonisation of disturbed habitats, such as minesites in Australia, by mycorrhizal fungi has been observed, but there is as yet insufficient information about the time required for this process to occur (Gardner & Malajczuk 1988, Jasper et al. 1992, Brundrett et al. 1995).
3. Soil Fertility
A key factor which effects the potential for mycorrhizas to benefit plants in particular sites is the supply of phosphate and nitrogen in soil (Abbott & Robson 1991, Grove et al. 1991). Phosphorus is generally considered to be the most important plant-growth limiting factor which can be supplied by mycorrhizal associations, because of the many abiotic and biotic factors which can restrict its mobility in soils (Harley & Smith 1983, Hayman 1983, Marschner 1986, Bolan 1991, Handreck 1997). Reductions in the benefit provided by mycorrhizal associations to plants are caused by increasing soil phosphorus levels (Bougher et al. 1990, Jones et al. 1990, Schweiger et al. 1995). High rates of P and N fertilizers suppress ectomycorrhiza development in the field (Menge et al. 1977, Newton & Pigott 1991). High concentrations of soil N can influence the relative abundance of different ECM types (Alexander & Fairley 1983).
4. Adverse Soil Conditions
Land degradation due to salinity, waterlogging, erosion, etc. are serious and growing problems in Australia and other countries (Bell 1988, Scott 1992). Excessive NaCl levels in soil inhibit mycorrhizal formation and restrict the activity of most mycorrhizal fungi, but some can tolerant these conditions (Malajczuk et al. 1989, Juniper & Abbott 1993). Observations in natural ecosystems have shown that plants with mycorrhizal associations are often less common than non-mycorrhizal species in soils which are waterlogged or saline, but that some mycorrhizal plants are normally present in even the worst soils (Brundrett 1991). ECM fungi can be highly sensitive to waterlogging of soils, while VAM fungi may be less sensitive (Theodorou 1978, Lodge 1989, Bougher & Malajczuk 1990).
5. Characteristics of Fungal Isolates
 |
Populations of mycorrhizal fungi are thought to have occupied the same soil habitats for millions of years, slowly adapting to changes in site conditions (Trappe & Molina 1986, Brundrett 2002). Some mycorrhizal fungi appear to have worldwide distribution patterns and have apparently adapted to a wide range of habitats, but soil factors such as pH restrict the distribution of other taxa. The fact that mycorrhizal research has often been concerned with plant growth responses with little consideration of the fungi involved has also helped to create the false impression that fungi are functionally equivalent (Abbott & Robson 1991, Brundrett 1991). Properties of individual mycorrhizal fungi result from their adaptations to soil/environmental/host factors. Some Properties of fungi considered to be important are (i) the amount of soil hyphae produced relative to root colonization, (ii) the rates of hyphal growth and root colonisation and (iii) physiological characteristics which regulate nutrient absorption or nutrient translocation by hyphae and exchange with the host (Kottke & Oberwinkler 1986, Smith & Gianinazzi-Pearson 1988, Abbott et al. 1992). |
 |
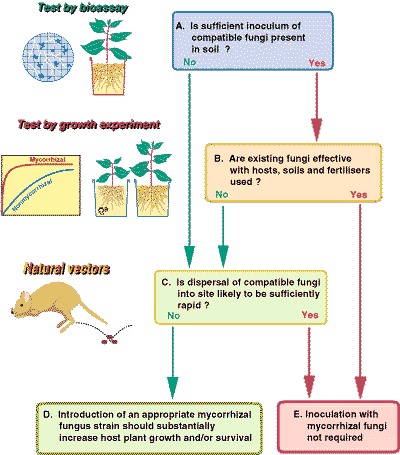
F. Manipulating Mycorrhizal Fungi
The potential for manipulating mycorrhizal associations to increase productivity in plantation forestry, or plant establishment during ecosystem recovery after severe disturbance, are the focus of major research initiatives. There is also much interest in their potential utilisation in agriculture and horticulture. However, it could be argued that we do not know enough about the role of mycorrhizal associations in natural, disturbed, or managed ecosystems to evaluate their potential for applied use.
The diagram provided below, outlines the steps in a decision making processes that can be used to determine if mycorrhizal fungi that are compatible with host plants are present in soils, or are likely to be introduced by natural mechanisms. This information is required to evaluate the potential benefits of mycorrhizal inoculation in a specific situation. There would only be a need to manipulate mycorrhizal fungi by inoculating plant seedlings or changing site management practices if substantial benefits from these manipulations are expected. Methods for the production of mycorrhizal plants in the glasshouse and nursery to allow experimentation or for use in the field are described in the book Working with Mycorrhizas in Forestry and Agriculture.
Version 2 © Mark Brundrett 2008