MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 6. NONMYCORRHIZAL PLANTS
A. Introduction
The definition which distinguishes nonmycorrhizal (NM) plants from those with facultative (variable) mycorrhizal associations, specifies that roots of NM plants are highly resistant to mycorrhizal fungi and normally remain uncolonised (see Section 7). Inconsistent use of definitions can contribute to uncertainty about which plants are nonmycorrhizal (see below). However, in most cases plants within a family share consistent nutritional strategies as discussed in the section on evolution.
The major table below lists flowering plant families where the majority of species examined were NM and knowledge of their alternative uptake mechanisms is briefly summarised in the subsections that follow. The table also includes estimated numbers of species for each NM family, in total and in Western Australia (WA), an ancient landscape with many NM species. Information in this table is designed to be used in conjunction with information on nonflowering plants, ectomycorrhizal plants, mycorrhizal Australian plants and mycorrhizal definitions provided elsewhere on this site.
Nonmycorrhizal plants are a major topic of study in Plant Biology (formerly Botany) at the University of Western Australia. In particular, Professor John Pate (retired) and his colleagues published many papers on parasites and carnivores and other aspects of plant nutrition. Currently the nutrition of plants with root clusters is a major topic of study by Professor Hans Lambers and his colleagues who are keen to extend this work to other West Australian plants with highly specialised nutritional strategies. Our School manages a nature reserve at Yule Brook where studies of carnivores, parasites, or plants with root clusters are ongoing (and where some picture on this page were taken). This reserve is also used for teaching to provide undergraduate students with the opportunity to see many of the amazing plants illustrated below.
| Topic | Major Sources |
| Definitions | Brundrett 1991, 2004, 2009 |
| Parasites | Parasites Website (Dan Nickrent), Kuijt 1969, Press & Graves 1995 |
| Carnivores | Juniper et al. 1989 |
| Root clusters | Lamont 1982, Skene 1988, Lambers et al. 2006 |
| Diversity | Brundrett 2009, Parasites Website, Haywood et al. 2006 (World), Florabase (WA Data) |
B. Nonmycorrhizal Flowering Plant Families (see Brundrett 2009 for updated list)
| Family | Genera | Spp. WA | Spp. World | Habit / Habitat | Category | Notes and References |
| I. ANGIOSPERMS - DICOTYLEDONS | ||||||
| Aizoaceae (see Mesembryanthemaceae also) | Carpobrotus, Pharnaceum, Sesuvium, Tetagonia, Trianthema, etc. | 50 (17) | 170 (12) | herbs | NM | Most plants NM - Brockhoff & Allaway 1988, Logan et al. 1989, Koske et al. 1992, Allsopp & Stock 1993, Brundrett et al. 1995, Dhillion et al. 1995 |
| Amaranthaceae | Alternanthera, Amaranthus, Celosia, Digeria, Gomphrena, Notorichium, Ptilotus, etc. | 140 (11) | 1000 (70) | herbs, shrubs | NM | Harley & Harley 1987, Tester et al. 1987, Koske et al. 1992, Reddell & Milnes 1992, Brundrett et al. 1995, O'Connor et al. 2001. NM or VAM - Ragupathy & Mehadevan 1993. |
| Apodanthaceae | Apodanthes, Pilostyles, Berlinianache | 3 (1) | 23 (3) | internal | Holoparasites | Family of obligate parasites lacking roots - Heywood et al. 1996 |
| Balanophoraceae | Balanophora | 0 | 43 (17) | internal | Holoparasites | Family of obligate parasites lacking roots - Heywood et al. 1996 |
| Brassicaceae (Cruciferae) |
Alyssum, Brassica, Cakile, Capsella, Blennodium, Heliophylla, Lepidium, Sisymbrium etc. | 101 (30) | 3350 (340) | herbs | NM | No arbuscules observed in 649 taxa inoculated with a VAM fungus, but 19% had hyphae and vesicles (DeMars & Boerner 1996). Some literature reports suggest VAM occurs occasionally, but most found NM roots only - Hirrel et al. 1978, Trappe 1981, Harley & Harley 1987, Allsopp & Stock 1993, Khan & Belik 1995, DeMars & Boerner 1996. |
| Byblidaceae | Byblis | 1 (3) | 1 (6) | herbs | Carnivore | Carnivorous plants - likely to be NM due to nutrition. |
| Capparaceae | Capparis etc. | 16 (3) | 470 (17) | herbs, shrubs, trees | NM | NM (most samples) - Khan, 1974, Trappe 1981, Koske et al. 1992, Muthukumar & Udaiyan 2000. Also expected to be NM due to phylogeny (sister to Brassicaceae, Cleomaceae). |
| Caryophyllaceae | Agrostemma, Arenaria, Cerastium, Calobanthus, Dianthus, Lychnis, Minuartia, Sagina, Silene, Stellaria, etc. | 41 (17) | 2200 (86) | herbs, shrubs | NM | Most samples NM - Maeda 1954, Harley & Harley 1987, Berch et al. 1988, Pawlowska et al. 1996, Johnson & Ryan 2000, Onipchenko & Zobel 2000, Fontenla et al. 2001, Hildebrandt et al. 2001, Cripps & Eddington 2005. |
| Cephalotaceae | Cephalotus | 1 (1) | 1 (1) | herb | Carnivore | Carnivorous plant - likely to be NM due to nutrition. |
| Ceratophyllaceae | Ceratophyllum | 1 (1) | 1 (3) | herbs | Aquatic | Free-floating submerged aquatic plants lacking roots. |
| Chenopodiaceae | Atriplex (AM)*, Enchyleana, Rhagodia, Salsola, Sclerolaena, etc. | 209 (28) | 1200 (97) | herbs, shrubs | NM (AM) | Predominalty NM famility. NM roots, or with very slight colonisation - Khan 1974, Hirrel et al. 1978, Trappe 1981, Harley & Harley 1987 - Pendleton & Smith 1983, Koske et al. 1992, Ragupathy & Mehadevan 1993, Dhillion et al. 1995, Khan & Belik 1995, Hildebrandt et al. 2001, O'Connor et al. 2001. *Atriplex AM - Williams et al. 1974, Asghari et al. 2005. |
| Cleomaceae (Capparaceae) | Cleome, etc. | 16 (3) | 310 (11) | herb | NM | NM roots - Pendleton & Smith 1983, Brundrett et al. 1995. Close realtives of the Brassicaceae and Capperaceae. |
| Convolvulariaceae (Cuscutaceae) | Cuscuta Bindweeds | 3 (1) | 170 (1)* | climbers | Hemiparasites | Hemiparasites and Holoparasites with branch haustoria - Molau 1995, Heywood et al. 2006. *Only parasites NM (lack roots at maturity), other members of this large family have VAM. |
| Crassulaceae | Cotyledon, Crassula, Sedum, etc. | 18 (5) | 1200 (33) | herb | NM / VAM* | 9 / 12 spp. NM - Harley & Harley 1987. Most samples have NM roots (VAM in only 1 of 20 samples from 8 spp.) - Allsopp & Stock 1993. NM - Onipchenko & Zobel 2000, Cripps & Eddington 2005. VAM in cultivated plants - Muller et al. 1994. *Apparently includes VAM and NM plants - More sampling required. |
| Cynomoriaceae | Cynomorium | 0 | 2 (1) | internal | Holoparasites | Very small family of obligate parasites lacking roots - Heywood et al. 1996. |
| Cytinaceae (Rafflesiaceae) | Bdallophyton, Cytinus | 0 | 7 (2) | internal | Holoparasites | Small family of obligate parasites lacking roots - Heywood et al. 1996. |
| Droseraceae | Aldrovanda (Waterwheel Plant), Drosera (Sundews), Dionaea (Venus Flytrap) | 105 (2) | 170 (3) | herbs, climbers, aquatic plant | Carnivores | Droseras are carnivorous plants with NM roots - Sward 1978, Brundrett & Abbott 1991, Koske et al. 1992, Brundrett et al. 1995, Fuchs & Haselwandter 2004, Weishampel & Bedford 2006 (hyphae but no arbuscules in last 2 reports). Some samples with VAM - Harley & Harley 1987, Maeda 1954, Cooke & Lefor 1998. Aldrovanda lacks roots. |
| Drosophyllaceae | Drosophyllum | 0 | 1 (1) | herbs | Carnivore | Carnivorous plants likely to be NM. |
| Eremolepidaceae (Santalaceae) | Antidaphne, Eubrachion, Lepidoceras Catkin-mistletoes | 0 | 12 (3) | epiphytes | Holoparasites | Holoparasites with branch haustoria on trees - Molau 1995, Heywood et al. 2006. No true roots. |
| Fabaceae (Papilionacea, Mimosaceae, Caesalpiniaceae) | Daviesia, Lupinus, Kennedia, Viminaria | 95 (2)* | >300 (3) | shrubs climbers |
NM | *Number in WA includes all Daviesia which may not be correct, only some Kennedia spp. are NM. cluster roots VAM or NM - Lamont 1982, Brundrett & Abbott 1991. *Total family size in WA = 1413 (103), but most Fabaceae roots have VAM and/or ECM. |
| Frankeniaceae | Frankenia | 27 (1) | 80 (4) | shrub | NM | NM - Selivanov & Eleusenova 1974, Harley & Harley 1987. Sparse colonisation, arbuscules not seen - O'Connor et al. 2001. |
| Hydnoraceae | Hydnora, Prosopanche | 0 | 15 (2) | herbs | Holoparasites | Holoparasites with root haustoria - Molau 1995, Heywood et al. 2006. Plants highly likely to be NM due to highly modified roots. |
| Hydrophyllaceae | Hydrolea, Hydrophyllum, Phycelia? | 3 (2) | 300 (15) | herbs, shrubs | NM (VAM)* | Hydrophyllum NM - McDougall & Liebtag 1928, Brundrett & Kendrick 1988, Hydrolea NM - Ragupathy & Mahadevan 1993. Phacelia VAM - Cripps & Eddington 2005 or NM Currah & Van Dyk 1986. *Family requires further investigation. |
| Krameriaceae | Krameria Rhatany | 0 | 18 (1) | herbs, shrubs | Hemiparasites (VAM) |
Root haustoria on other plants - Molau 1995, Heywood et al. 2006. Some VAM in roots - Lesica & Antibus 1986, Carillo-Garcia 1999. |
| Lauraceae | Cassytha (Dodder) | 15 (1)* | 20 (1)* | vines | Hemiparasites | Climbing haustorial parasite without roots. *The Lauraceae also have many non-parasitic VAM plants not included in these totals. |
| Lennoaceae | Lennoa, Pholisma | 0 | 5 (2) | herbs | Holoparasites | Holoparasites with branch or root haustoria - Molau 1995, Heywood et al. 2006. Likley to be NM due to nutritinal mode. |
| Lentibulariaceae | Genlisea, Pinguicula, Utricularia (butterworts, bladderworts) | 39? (1) | 320 (3) | herbs | Carnivores | Aquatic and semi-aquatic carnivorous plants- Lowrie 1998. NM - Maeda 1954, Harley & Harley 1987, Cheers 1992, Muthukumar & Udaiyan 2000. Genlisea - no data. |
| Loranthaceae | Amyema, Decaisnina, Dendrophthoe, Lysiana, etc. (Mistletoes) Nuytsia (WA Christmas Tree) | 36 (6) | 906 (73) | epiphytes, tree | Hemiparasites | Parasitic: epiphytes attached to branches by haustoria, or trees with root haustoria (e.g. Nuytsia). |
| Mesembryanthemaceae (Aizoaceae s.l.) | Carpobrotus, Drosanthemum, Lampranthus, Mesembryanthemum, Ruschia, etc. | * | 123 (1680) | shrubs | NM | All roots sampled NM (43 samples / 12 spp.) - Allsopp & Stock 1993. Included in Aizoaceae in some surveys. *WA plants classified in Aizoaceae. |
| Misodendraceae | Misodendron Feathery Mistletoe | 0 | 10 (1) | epiphytes | Hemiparasites | Pparasites with branch haustoria on Nothofagus - Molau 1995, Heywood et al. 2006. NM plants lacking roots. |
| Mitrastemonaceae | Mitrastemon | 0 | 2 (1) | herbs | Holoparasites | Endoparasitic in roots of Fagaceae - Molau 1995, Heywood et al. 2006. NM plants lacking roots. |
| Myricaceae | Comptonia, Myrica | 0 | 62 (4) | shrubs, trees | Cluster Roots | ECM would be expected due to position of the family in the Fagales, but most recent studies found roots to be NM, or less often to have VAM (Berliner & Torrey 1989, Semones & Young 1995, Wang & Qui 2006, Gemma & Koske 1997, Hurd & Schwintzer 1997). Nitrogen fixing associations (Frankia) |
| Nepenthaceae | Nepenthes | 0 | 85 (1) | climbers | Carnivores | NM - Janse 1896 (cited in Janos 1987). Carnivorous plants likely to be NM. |
| Nyctaginaceae | Boerhavia, Bouganvillea, Mirabilis, Pisonia | 11 (4) | 350 (30) | trees, shrubs | NM* (ECM) | NM - Koske et al. 1992. *More sampling required. Pisonia grandis has ECM. |
| Olacaceae (part only) |
Olax, Ximenia | 6 (1) | 104 (14)* | trees, shrubs | Hemiparasites | *Total excludes autotrotrophic plants in family. NM - Ragupathy & Mehadevan 1993. Onguene & Kuyper 2001 found VAM (with arbs?) in a hemiparasite. Hemiparasites with root haustoria - Kuijt 1969, Heywood et al. 2006. Expected to be NM due to nutrition. |
| Opiliaceae | Cansjera, Opilia, etc. | 2 (2) | 34 (11) | trees, vines | Hemiparasites | Parasites with root haustoria - Molau 1995, Heywood et al. 2006. Probably NM. |
| Orobanchaceae (Scrophulariaceae) | Hemiparasites: Castilleja, Cordylanthus, Euphrasia, Melampyrum, Orthocarpus, Pedicularis, Rhinanthus etc. Holoparasites: Epifagus, Orobanche, etc. | 9 (3) | 2062 (90)* | herbs | Hemiparasites, Holoparasites | *Parasitic ex. Scrophulariaceae are now placed in the Orobanchaceae (Nickrent 2007). Hemi-parasites NM - Lesica & Antibus 1986 (27 species), Onipchenko & Zobel 2000, Cázares et al. 2005, but some apparently have VAM also - Pawlowska et al. 1996. Holoparasitic plant NM - Brundrett & Kendrick 1988. |
| Papaveraceae (Fumariaceae) |
Argemon, Chelidonium, Corydalis, Dicentra, Sanguinaria, etc. | 0 | 760 (41) | herbs | NM , VAM | Selivanov & Eleusenova 1974, Pendleton & Smith 1983, Harley & Harley 1987, Berch et al. 1988, Brundrett & Kendrick 1988 - VAM and NM. |
| Phytolaccaceae | Phytolacca | 0 | 31 (4) | trees, shrubs, climbers, herbs | NM* | NM - Gerdemann 1968, Janos 1980, Schwab & Leonard 1984. Straker et al. 2007 - hyphal colonization without arbuscules. *More sampling required for family status. |
| Polygonaceae | Amblygonum, Cocolobia, Eriogonum, Persicaria, Polygonum, Rumex etc. | 17 (3) | 1100 (47) | herbs, shrubs, trees, climbers | NM | Most reports are NM - Maeda 1954, Pendleton & Smith 1983, Berch et al. 1988, Koske et al. 1992, Ragupathy & Mehadevan 1993, Pawlowska et al. 1996, Cripps & Eddington 2005. Polygonum may also have VAM - Koske et al. 1992. Family includes many weeds. |
| Portulacaceae | Calandrinia, Claytonia, Montia, Portulaca | 48 (5) | 500 (25) | herbs, shrubs, trees | NM (VAM) | NM (most samples) - Tester et al. 1987, Berch et al. 1988, Brundrett & Kendrick 1988, McGee 1986, Brundrett et al. 1995, Dhillion et al. 1995, O'Connor et al. 2001. Lewisia VAM - Cripps & Eddington 2005. |
| Proteaceae | Banksia, Dryandra, Grevillea, Hakea, Protea, Stenocarpus, Telopea, etc. | 758 (17) | 1700 (80) | shrubs, trees | NM Cluster Roots |
Roots NM in Australia and South Africa - Allsopp & Stock 1993. Many have cluster roots. |
| Rafflesiaceae |
Rafflesia, Rhizanthes, Sapria | 0 | 30 (3) | internal | Holoparasites | Internally growing parasitic plants lacking roots at maturity (Kuijt 1969, Heywood et al. 1996) |
| Santalaceae | Arjona, Comandra, Exocarpus, Leptomeria, Myoschilos, Quinchamalium, Santalum, Thesium | 27 (6) | 490 (35) | trees, shrubs, herbs | Hemiparasites (VAM) |
A familiy of Hemiparasites with root haustoria, or occasionally branch haustoria (Kuijt 1969, Lamont 1982, Cernusak et al. 2004, Heywood et al. 1996). Most studies found roots to be NM - Lesica & Antibus 1986, Dhillon et al. 1995, Pawlowska et al. 1996, Fontenla et al. 2001. VAM also reported - Currah & Van Dyk 1986, Koske et al. 1992, Allsopp & Stock 1993, Ragupathy & Mehadevan 1993. Expected to be NM due to nutrition. |
| Sarraceniaceae | Darlingtonia, Heliamphora, Sarracenia | 0 | 20 (3) | herbs | Carnivores | Carnivorous plants with pitfall traps likely to be NM. |
| Schoepfiaceae (Oleaceae) | Arjona, Quinchamalium, Schoepfia | 0 | 50 (3) | shrubs | Hemiparasites | Small family of parasites. Likely to be NM. |
| Viscaceae | Arceuthobium, Phoradendron, Viscum, etc. (Mistletoes) | 5 (2) | 546 (7) | epiphytes | Hemiparasitic | Family of parasites with haustorial connections to tree branches (Kuijt 1969). |
| Zygophyllaceae | Larrea, Peganum, Tribulus, Zygophyllum | 38 (4) | 275 (24) | herbs, shrubs | NM | Khan 1974, Trappe 1981, Tester et al. 1987, Pendleton & Smith 1983, Peterson et al. 1985, Koske et al. 1992, Allsopp & Stock 1993, O'Connor et al. 2001 |
| II. ANGIOSPERMS - MONOCOTYLEDONS | ||||||
| Centrolepidaceae | Centrolepis | 21 (2) | 35 (3) | sedge-like | Aquatic | Sward 1978, Brundrett unpublished |
| Commelinaceae | Cartonema, Commelina, Murdannia, Tradescantia, etc. | 9 (4) | 650 (40) | herbs | NM (VAM) | NM - Tester et al. 1987, Logan et al. 1989, Brundrett et al. 1995. Most samples NM - da Silva et al. 2001, Muthukumar & Udaiyan 2000. VAM - Khan & Belik 1995. |
| Cymodoceaceae | Amphibolis, Cymodocea, Halodule, Syringodium, Thalassodendron | 2 (2) | 16 (5) | herbs | Marine Plants | NM Marine plants (Nielson et al. 1999). |
| Cyperaceae | Arthrostylis, Bulbostylis, Carex, Cyperus, Eleocharis, Fimbristylis, Rhynchospora, Scleria, etc. | >330 (31) | 4500 (100) | sedges | NM Sand-binding Dauciform |
Dauciform roots in some genera - Davies et al. 1973, Shane et al. 2005. Sand binding roots also present in WA. NM - Maeda 1954, Powell 1975, Brundrett & Kendrick 1988, Logan et al. 1989, Pawlowska et al. 1996, Bellgard 1991, Onipchenko & Zobel 2000, Cornwell et al. 2001, Fontenla et al. 2001, Miller 2005, Weishampel & Bedford 2006. Inconsistently mycorrhizal - Koske et al. 1992, Meney et al. 1993, Miller et al. 1999, da Silva et al. 2001, Fontenla et al. 2001, Muthukumar et al. 2004, Gai et al. 2006, Perrier et al. 2006. |
| Dasypogonaceae | Kingia | 53 (8)* | 16 (4)* | shrub | Sand-binding | *Genera included in family varies. NM roots with long root hairs - Brundrett & Abbott 1991 |
| Haemodoraceae | Anigozanthos, Conostylis, Haemodorum | 82 (8) | 100 (13) | herbs | Sand-binding | Brundrett & Abbott 1991, Brundrett et al. 1995. Sand binding roots with very long root root hairs - Pate & Dixon 1996. |
| Hydatellaceae | Hydatella, Trithuria | 8 (2) | 10 (2) | herbs | Aquatic* | Likley to be nonmycorrhizal. *More sampling required. |
| Hydrocharitaceae | Halophila, Thalassia | 6 (2)* | 16 (2)* | herbs | Marine* Plants | The seagrasses Thalassia are Halophila NM - Nielson et al. 1999, Brundrett & Cambridge unpublished. *Marine plants likley to be nonmycorrhizal. Freshwater aquatic members of family have VAM when conditions are suitable - Khan & Belik 1995. |
| Juncaceae | Juncus, Luzula | 24 (2) | 440 (7) | rushes | NM (VAM) | Root clusters occur in some species of Juncus - Davies et al. 1973, Shane et al. 2005. NM - Sward 1978,Väre et al. 1997, Onipchenko & Zobel 2000, Fontenla et al. 2001. Hildebrandt et al. 2001. Alpine Luzula sp. 0-61% VAM - Johnston & Ryan 2000. |
| Juncaginaceae | Triglochin | 15 (1) | >20 (4) | Aquatic herbs | NM* | NM - Belik & Khan 1993, Fontenla et al. 2001, Hildebrandt et al. 2001. *Needs more sampling. |
| Najadaceae | Najas | 6 (1) | 40 (1) | aquatic herbs | NM* | Khan & Belik 1995, Brundrett et al. 1995 *Needs more study |
| Posidoniaceae | Posidonia | 8 (1) | 10 (1) | aquatic herbs | Marine Plants | NM - Brundrett & Cambridge unpublished |
| Restionaceae | Alexgeorgia, Hypodiscus, Ischyrolepis, Lyginia, Leptocarpus, Loxocarya, Staberoha, Thamnochortus, Willdenowia, etc. | 101 (27) | 500 (50) | rushes | NM Sand Binding Capillaroid |
NM in Australia - Brundrett & Abbott 1991, Reddell & Milnes 1992, Brundrett et al. 1995 and South Africa - Allsopp & Stock 1993, or VAM occasionally present - Meney et al. 1993. They often have capillaroid root clusters, or sand binding roots with very long root hairs, as shown below - Lamont 1982, Pate & Dixon 1996, Shane et al. 2005. |
| Xyridaceae | Xyris | 17 (1) | 300 (5) | herbs | NM* | Brundrett et al. 1995. *Needs more sampling. |
| Zosteraceae | Heterozostera, Phyllospadix, Zostera | 2 (2) | 18 (4) | herbs | Marine Plants | Zostera NM - Nielson et al. 1999. Marine plants unlikley to be mycorrhial (Brundrett 1991). |
| Key to Row Shading for Plant Families | |||||
| VAM plants also | Cluster or Dauciform Roots | Parasites | Carnivores | Marine Plants | Other NM Plants |
- Abbreviations
- NM = nonmycorrhizal, WA = Western Australia, Numbers in brackets = genera, Families with both VAM and NM plants are shaded green.
- Excluded Families
- Excluded families listed by Wang & Qui (2006) that are known to have or probably have mycorrhizal roots, or were only represented by a single record. As stated by Harley & Harley (1987), single reports are not sufficiently reliable to make a diagnosis about the presence or absence of mycorrhizas for a family: Adoxaceae, Bataceae, Cannaceae, Cyclanthaceae, Erythroxylaceae, Loasaceae, Nymphaeaceae.
- Doubtful Families
- These include plants known to be NM, but the status of the whole family is in doubt due to conflicting evidence or insufficient sampling: Crassulaceae, Frankeniaceae, Hydrophyllaceae, Papaveraceae, Phytolaccaceae, Plumbaginaceae, Hydatellaceae, Saxifragaceae, etc.
- Plant family classification
- Mostly consistent with Heywood et al. (2007) - Flowering Plant Families of the World published by the Royal Botanic Gardens, Kew. Families of parasitic plants follow the online list provided by Dan Nickrent of Southern Illinois University (7/5/07).
- Numbers of taxa
- Numbers of Western Australian (WA) taxa are from the West Australian Herbarium census of plants published in June 2007, with weeds excluded. Estimated total numbers of taxa are from Heywood et al. (2007) and Dan Nickrent's website (2007).
1. Recognising Nonmycorrhizal (NM) Plants
Families listed above meet the definition of NM plants, at least for the majority of their members. However, in many of these families, there are occasional reports of VAM (usually lacking arbuscules, or with arbuscules present sporadically and often confined to older roots). However, the majority of species in NM families have roots which are highly resistant to mycorrhizal fungus hyphae. In the mycorrhizal literature, endophytic hyphae and vesicles of Glomeromycotan fungi were interpreted as VAM by some authors, but not by others (Hirrel et al. 1978, Brundrett 2006). Despite these inconsistencies, it is clear that some plant families are predominantly NM, and most of these families have been recognised for some time (e.g. Maeda 1954, Gerdemann 1968, Selivanov & Eleusenova 1974, Trappe 1981, Harley & Harley 1987, Tester et al. 1987, Brundrett 1991, Molina et al. 1992, Allsopp & Stock 1993, Schreiner & Koide 1993, Cripps & Eddington 2005), but others are reported here for the first time (Brundrett in prep.). Plants with roots that are unlikely to be mycorrhizal due to the habitats where they grow are listed below.
- NM non-flowering plants such as mosses are summarised in Section 2.
- Epiphytes such as ferns are often NM in trees, but the same species can be mycorrhizal in soil (Nadarajah & Nawawi 1993). The mycorrhizal status of epiphytes is poorly known, but mycorrhizas are reported in some cases (Lesica & Antibus 1990, Janos 1993, Gemma & Koske 1995).
- Hydrophytes are often NM (Maeda 1954, Beck-Nielsen & Madsen 2001, Kai & Zhiwei 2006, Radhika & Rodrigues 2007). Khan & Belik (1995) list aquatic plants that may be NM or have VAM roots in different habitats, including aquatic members of the Alismataceae, Butomacaea, Araceae, Haloragaceae, Nymphaeaceae, Podostemonaceae, Pontederiaceae, Potamogetonaceae and Typhaceae.
- The most highly specialised hydrophytes, floating plants with few or no roots, are unlikely to ever be mycorrhizal (e.g. Ceratophyllum). Floating aquatic plants such as Azolla, Eichhornia, Lemna and Marsilea lack roots and are also NM (Maeda 1954, Ragupathy & Mahadevan 1993, Beck-Nielsen & Madsen 2001, Kai & Zhiwei 2006, Radhika & Rodrigues 2007).
- Seagrasses (Cymodoceaceae, Hydrocharitaceae, Posidoniaceae, Zosteraceae) are NM (Nielsen et al. 1999, Brundrett & Cambridge unpublished).
- Mangroves (Avicenniaceae, Posidoniaceae, Rhizophoraceae) are reported to have VAM in one study, but not in 3 others (Maeda 1954, Rose 1981, Mohankumar & Mahedevan 1986, Sengupta & Chaudhuri 2002).
- Plants in families that tend to occur in extremely harsh habitats can be NM in some locations and mycorrhizal in others, presumably due to variations in soil conditions. These severe conditions include recently disturbed habitats (e.g. sand dunes), very cold habitats (arctic and alpine), saline soils and very arid habitats (see Brundrett 1991).
Many of the NM plants listed in the table above have alternative nutrient uptake mechanisms such as carnivory, parasitism, or roots with specialised morphologies and branching, such as the cluster and dauciform roots described below. Roots of NM plants also tend to be highly branched, with fine lateral roots and long root hairs as described in the mycorrhizal dependency section and illustrated for Drosera below.
C. Plants with Cluster Roots
One of the largest groups of NM plants have cluster roots - dense aggregations of lateral roots with long root hairs. These include many Proteaceae members, especially those occurring in highly infertile soils of Western Australia and South Africa. NM cluster roots also occur in the Myricaceae and some genera of the Fabaceae (i.e. Lupinus). Cluster roots occur with mycorrhizas in Viminaria and Aspalanthus of the Fabaceae and members of the Betulaceae, Casuarinaceae and Eleagnaceae (Skene 1998, Diem et al 2000, Lambers et al. 2006).
 |
 |

|
Banksia menziesii |
Daviesia decurrens a NM pea (Fabaceae) with cluster roots. |
Hakea victoria |
Cluster roots promote nutrient uptake by their large surface area and production of exudates that increase nutrient solubility (Lambers et al. 2006). Cluster roots often form a dense mat near the soil surface in contact with decomposing litter (Lamont 1993), support populations of P-solubilising bacteria (Wenzel et al. 1994), secrete organic acids and are very efficient at P adsorption (Grierson 1992, Shane et al. 2003, 2004). These mechanisms have been shown to increase the availability of nutrients such as phosphorus that are immobilised in soils (Shane et al. 2006). The greatest diversity and abundance of the Proteaceae occurs in highly infertile soils with a high P-fixing capacity, where it is expected that cluster roots are most advantageous (Pate et al. 2001, Lambers et al. 2006). Proteaceae species also occur in less nutrient limited habitats, such as rainforests.
Rollover images show greater details.
Roots of members of the Proteaceae, are usually highly resistant to mycorrhizal fungi, even when exposed to high inoculum levels (e.g. Brundrett & Abbott 1991), due to mechanisms that are poorly understood. The occasional occurrence of VAM in roots of the Proteaceae (Bellgard 1991, Boulet & Lambers 2005) requires further study. The Proteaceae are dominant species in some habitats and provide important sources of food to nectar-feeding birds and mammals, as shown below. Unfortunately, members of this family, which contains many rare species, are also high susceptible to Phythophthora dieback disease (Shearer et al. 2007).
D. Root Clusters in Monocotyledons
The Cyperaceae, Restionaceae and Juncaceae are generally considered to by NM families (Newman & Reddell 1987, Tester et al. 1987, Brundrett 1991, Miller 2005). However, there also are reports of VAM in sedges - especially hyphae and vesicles, sometimes with occasional arbuscules (Miller et al. 1999, Muthukumar et al. 2004, Gai et al. 2006). It is not clear if the very large plant family Cyperaceae includes both NM and VAM species, or if mycorrhizal colonisation is sometimes regulated by environmental factors. The diagnosis of roots with inconsistent associations is complicated by inconsistent applications of definitions in surveys. Sedges typically have fine roots with long roots hairs that provide an alternative to mycorrhizas (Brundrett & Kendrick 1988, Miller et al. 1999).
1. Dauciform Roots in Sedges
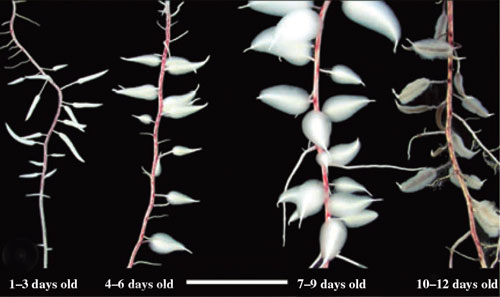
Some members of the Cyperaceae have specialised swollen "dauciform" roots (Davies et al. 1973, Lamont 1974, Meney et al. 1993, Pate & Dixon 1996, Shane et al. 2006). These have been found in species of Carex, Cladium, Cyathochaeta, Gahnia, Kobresia, Lepidosperma and Schoenus (Davies et al. 1973, Shane et al. 2005). Dauciform roots function in a similar manner to cluster roots by exuding organic acids to facilitate nutrient uptake from soils (Shane et al. 2006).
 |
 |
Four stages of dauciform root development in Schoenus unispiculatus (see Shane et al. 2006). Very abundant long root hairs developed in 7–9 days. Young roots (1–3 days old) have a distinct ‘carrot shape’ on a slender stalk. Dauciform roots senesced after 10–12 days (white bar is 1.2 cm). Image on right shows greater detail of dauciform roots. (Photos by Mike Shane) | |
2. Capillaroid Roots - Root Clusters of Rushes
Root clusters in rushes (Restionaceae, Juncaceae) differ in structure from the swollen (dauciform) laterals of sedges. These root clusters, with dense aggregations of lateral roots with long root hairs, were named "Capillaroid Roots" by Lamont (1982), or cluster roots (Meney & Pate 1999). They have been found in members of the Restionaceae from West Australia and South Africa (Meney & Pate 1999, Shane et al. 2006). It has not been confirmed that they function in a similar manner to cluster roots and dauciform roots, but it seems likely.
3. Sand-binding Roots
Sedges, rushes and other NM monocots from Western Australia in the families Restionaceae, Cyperaceae, Haemodoraceae, Lomandraceae, etc. have sand-binding roots (Brundrett & Abbott 1991, Pate & Dixon 1996). As illustrated below, these roots have persistent sheaths of sand cemented by mucilage and root hairs. The role of these roots are unknown, but they are associated with the absence of mycorrhizas, so may have roles in nutrient uptake. Sand sheaths may also help protect long-lived roots.

|
 |
 |
Anigozanthos manglesii (Haemodoraceae) The Red and Green Kangaroo Paw The floral emblem of Western Australia. |
Anarthria scabra (Anarthriaceae) female inflorescence on a sedge like plant. |
Ecdeiocolea monostachya (Ecdeiocoleaceae) a sedge-like plant with sand-binding roots. |
E. Parasitic & Hemiparasitic Plants
Parasitic plants that acquire nutrients and water directly from other plants are unlikely to receive substantial benefits from mycorrhizas. Parasitic plants with root haustoria in the Santalaceae, Scrophulariaceae, etc. and Mistletoes in the Loranthaceae, Viscaceae, etc. are listed in the table above. There are over 4500 parasitic plants in total (Nickrent 2007), which is equivalent to about 1% of the World's flowering plants. The largest families of parasitic plants are the Olacaceae, Loranthaceae, Santalaceae and Orobanchaceae. The table above lists all known parasitic plants, as a category of NM plants.
Many holoparasites lack roots and the majority of hemiparasites where roots have been assessed are NM in families such as the Orobanchaceae (Scrophulariaceae), where Lesica & Antibus (1986) found 27 species all had ‹ 5% colonization without arbuscules. However, there are reports of VAM in hemiparasites in the Santalaceae and Krameriaceae (Lesica & Antibus 1986).
 |
 |
 |
Exocarpus sparteus (Santalaceae) a hemiparasitic shrub. |
Cassytha sp. (Lauraceae) Dodder - a climbing parasitic plant. |
Cassytha sp. haustoria on a eucalypt branch. |
Parasitic plants steal nutrients directly from other plants via haustoria, so their mycorrhizal associations (if any) are likely to be redundant. Most of the hemiparasitic plants that have been examined have NM roots (if any), and holoparasites lack normal soil-bourne roots. The amazing Western Australian (WA) "Christmas Tree" Nuytsia floribunda (Loranthaceae) provides an example of root haustoria below.
 |
 |
Nuytsia floribunda the West Australian Christmas Tree is a nonmycorrhizal parasitic tree with root haustoria. It has spectacular orange flowers, produced in profusion Nov-Dec in WA. |
Nuytsia root haustoria (white ovals 5-15 mm across attached to thin white roots). |
Nuytsia parasitises a wide variety of plants and is infamous for severing subterranean infrastructure such as communication cables and plastic irrigation pipes. | |
Australia also has a wide diversity of epiphytic mistletoes (Barlow & Wiens 1977) which also belong to the Loranthaceae family. In Australia host specialisation has resulted in morphological diversity as mistletoes that mimic leaves of their hosts (Barlow & Wiens 1977). Several contrasting examples of mistletoes are shown below.
 |
 |
Amyema preissii a mistletoe from WA that occurs on Acacia species. |
Amyema nestor a mistletoe from WA that occurs on Acacia species. |
F. Carnivorous Plants
Plants with highly specialised nutrient capture strategies usually have NM roots, as is the case of carnivorous plants in the genera Drosera, Utricularia and Aldrovanda (if they have roots). The nutritional functions of roots are replaced by insect traps in these species, so mycorrhizas have become redundant. Drosera spp. have roots that are very fine with very long root hairs (see below). Experiments have demonstrated that carnivorous plants acquire a substantial proportion of their nutrients by digestion of prey (Juniper et al. 1989, Schulze et al. 1997).
 |
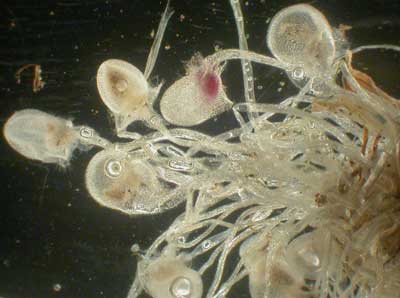 |
Utricularia menziesii an insectivorous bladderwort (Photo by Mike Shane). |
Traps produced at the base of a bladderwort (Utricularia multifida) to trap invertebrates. |
Plants with "snap traps" (Utricularia and Aldrovanda) that catch and digest small aquatic animals, lack normal roots at maturity (Cheers 1992, Lowrie 1998, Juniper et al. 1989, Adlassnig et al. 2005), so it is safe to conclude they are NM. Drosera and Pinguicula species have leaves that trap insects and roots which usually are NM. Less is known about mycorrhizas of insectivorous plants with "pitfall traps" where insects are digested (Sarracenia, Darlingtonia, Nepenthes, etc.), but it is expected they primarily obtain nutrients by carnivory. The unique Western Australian carnivorous plants Cephalotis follicularis (Cephalotaceae) and Byblis spp. (Byblidaceae), which are illustrated below, are also likely to be NM.
Carnivorous plants not listed in the table above because roots have not been examined include Brocchinia, Catopsis (Bromeliaceae) and Triphyophyllum (Dioncophyllaceae). Roridula gorgonias (Roridulaceae), a semicarnivorous plant endemic to South Africa, has VAM but also acquires nutrition from insects (Midgley & Stock 1998). Protocarnivory or semicarnivory has been proposed in other plants (e.g. Stylidium) but requires further investigation (Darnowski et al. 2006). It is likely that plants gradually lose mycorrhizas as they become more dependent on carnivory, so intermediate stages in this evolutionary pathway will have both nutritional strategies.
Assessment of the phylogeny of carnivorous plants has shown that Drosera, Dionaea, Aldrovanda, Drosophyllum, Nepenthes and Triphyophyllum are 2 closely related clades in the Caryophyllales (Cameron et al. 2002). Thus, most carnivorous plants belong to a single lineage of predominantly NM plants in an order including other NM families (Aizoaceae, Caryophyllaceae, Chenopodiaceae, Polygonaceae).

|
 |
 |
Utricularia multifida |
Sarracenia purpurea a carnivorous pitcher plant (Ontario, Canada). |
Drosera glanduligera a pygmy sundew (Western Australia). |
G. Conclusions
The evolution of nutrient uptake mechanisms, such as new types of mycorrhizas or NM cluster roots, coincided with the origin of many plant families which apparently became more competitive, or were able to occupy new habitats (Brundrett 2002). Plants that obtain nutrients by carnivory or parasitism, also tend to be NM. We would assume that these mechanisms provided a selective advantage due to increased nutrient uptake efficiency relative to association costs. However, analysis of the costs and benefits of root nutrient uptake mechanisms is complex, because mycorrhizal plants remain dominant in most habitats, while NM plants are more likely to occur in wet, saline, dry, or cold habitats, where plant productivity is low, or inoculum of mycorrhizal fungi could be scarce (Brundrett 1991).
Highly-leached soils in the ancient landscapes of Western Australia (WA) include many habitats where plants with NM roots tend to be more abundant than elsewhere in the World (Lamont 1982, Lambers et al. 2006). However, the importance of plants with ECM and VAM roots is also high in these habitats (e.g. the Myrtaceae and Fabaceae are often dominant). The Proteaceae (3rd in size) and Cyperaceae (7th in size) are two of the top ten families of plants in WA (the remaining 8 are mycorrhizal), a indication of the relative diversity of NM plants in these habitats.
| Further Examples of NM Plants from Western Australia | ||

Anarthria scabra Anarthriaceae |

Banksia coccinia |

Grevillea wilsonii |

Conostylis setigera |

Isopogon |

Kingia australis |
Terminology
A. Nonmycorrhizal Roots
- Root Clusters
- Dense aggregations of lateral roots such as cluster roots and capillaroid roots (Lambers et al. 2006).
- Cluster Roots
- A specific type of root clusters, where closely-spaced lateral roots are densely covered with root hairs. These occur in particular dicotyledon families (Proteaceae, Fabaceae, etc.). Cluster roots function by secretion of organic acids to solubilise mineral nutrients from infertile soils (Lambers et al. 2006).
- Dauciform Roots
- Swollen lateral roots produced by sedges (Cyperaceae) that are densely covered in root hairs (Lamont 1974). These are much thicker than normal lateral roots and spindle shaped (tapering), i.e. "carrot-shaped". They have a similar role to cluster roots in nutrient uptake (Shane et al. 2004).
- Capillaroid Roots
- A type of root cluster produced by rushes (Restionaceae) with exceptionally dense coverings of long root hairs (Lamont 1982).
B. Parasitic Plants
Brief definitions of parasitic and hemiparasitic plants are provided below. Dan Nickrent's website which should be consulted for more information.
- Holoparasite
- A nonphotosynthetic parasite that obtains all the water and nutrients required for sustenance from a host plant. These have connections with both the xylem and phloem of the host.
- Hemiparasite
- A parasite that is photosynthetic and may have roots, but obtains water and nutrients from one or more host plants. Most have haustoria that connect to xylem, but some also obtain photosynthates from the host phloem (e.g. dwarf mistletoes).
- Mistletoe
- Epiphytic hemiparasite attached to tree branches by haustoria.
- Obligate Parasite
- A plant that must attach to a host to complete its life cycle (some grow inside the host). All holoparasites are obligate, but some hemiparasites are not.
- Haustorium
- The structure (usually a modified root) connecting a parasite to its host(s). Haustoria physically penetrate the host and function by connecting its vascular tissue to the parasite.
Version 2 © Mark Brundrett 2008















