MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 2. EVOLUTION OF MYCORRHIZAS
A. Introduction
Mycorrhizal evolution is hypothesised to have progressed from endophytic to balanced symbiotic associations where both partners are interdependent due to the exchange of limiting resources (Brundrett 2002). Fossil Glomeromycotan associations and spores, as well as DNA-based phylogenies suggest mycorrhizas evolved with the first land plants. This evidence is used to identify mycorrhizal plant lineages and estimate their age in a summary provided below (after Brundrett 2002). Information on mycorrhizas of primitive plants has been updated with new references and more recent phylogenetic schemes for these plants.
| Topic | Major Sources |
| Evolution of mycorrhizas | Brundrett 2002 (New Phytologist) |
| Lower plant mycorrhizas | Many sources including: Maeda 1954, Read et al. 2000, Kottke & Nebel 2005 and Ligrone et al. 2007 |
| Fossil mycorrhizas | Pirozynski & Dalpé 1989, Tayor & Krings 2005 |
| Examples of Primitive Conifers from Western Australia | ||

Macrozamia dyeri |

Podocarpus drouynianus |

Podocarpus drouynianus |
B. Fossil History of Mycorrhizas
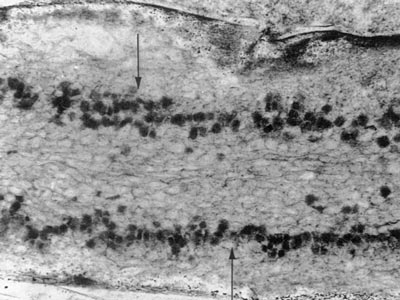
Fossils of early land plants contain fungal associations which are similar to arbuscular mycorrhizas in extant plants, as shown below, and ancient fossil spores of Glomeromycotan fungi are also known (Pirozynski & Dalpé 1989, Redecker et al. 2000, Dotzer et al. 2006, Strullu-Derrien & Strullu 2007). This story is described in detail in recent reviews (Brundrett 2002, Taylor et al. 2004, Taylor & Krings 2005), so only summary tables of land plant and fungus history are presented here. Photos of 400 Myr old fossil mycorrhizas are reproduced below courtesy of Professor Thomas Taylor at the University of Kansas.
 |
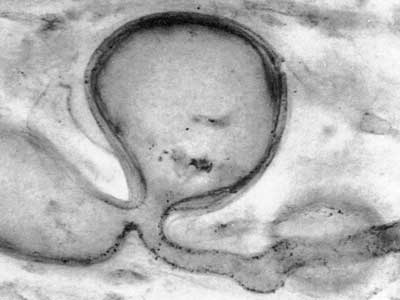 |
400 MY old fossil mycorrhiza-like association in Aglaophyton major rhizome (Taylor et al. 1995). |
Vesicle-like structures in Aglaophyton major rhizome (Taylor et al. 1995). |
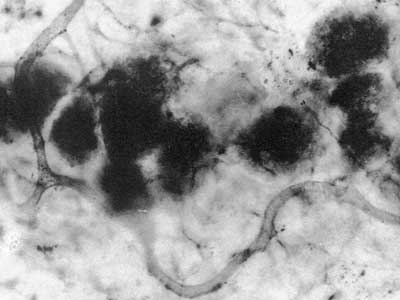 |
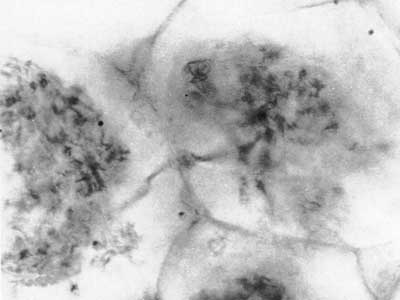 |
Arbuscule-like structures in Aglaophyton major major rhizome (Taylor et al. 1995). | |
Table 1. The mycorrhizal status of major plant lineages, with their approximate ages estimated using fossil evidence and molecular plant phylogenetics.
| Period | Plant | Mycorrhizas |
| Ordovician to Silurian (476-432 Myr) | The first bryophyte-like land plants | Limited fossils without roots, mycorrhizas unknown. |
| Ordovician (476 Myr) to Present | Liverworts and hornworts | Currently have VAM-like associations (with or without arbuscules), other associations, or are NM (see below) |
| Silurian (415-425 Myr) to Present | Mosses | No roots. Currently NM. Some have endophytic Glomeromycotan fungi (see below) |
| Devonian (400 Myr) | Aglaophyton major, Nothia aphylla and other early land plants of uncertain affinities | Fossils with VAM-like hyphae and arbuscules in rhizome or thallus (Taylor et al. 1995, Taylor & Krings 2005, Krings et al. 2007) |
| Devonian (395 Myr) to Present | Lycopods: Lycopodium, Selaginella, etc. | VAM in sporophyte, underground gametophyte MH VAM (see below) |
| Devonian (395 Myr) to Present | Sphenophytes: Equisetum, etc. | Living Equisetum species have VAM (see below) |
| Mid Devonian (385 Myr) | Sphenophytes, Lycopods, Pteridophytes | First plants with roots resembling those of modern plants |
| Mid Devonian (385 Myr) to Present | Pteridophytes (Ferns) | Most living ferns have VAM (see below) |
| Mid Devonian to Present | Cycads | VAM (see below) |
| Triassic (215-235 Myr) | Cycad from Antarctica (Antarcticycas sp.) | Earliest fossil VAM association in roots? (Phipps & Taylor 1996). |
| Permian (265 Myr) to Present | Ginkgoales: Ginkgo, etc. | Extant tree has VAM (see below) |
| Triassic (235 Myr) to Present | Southern Hemisphere conifers: Araucariaceae, Podocarpaceae | Living trees have VAM (see below) |
| Early Jurassic (190 Myr) to Present | Northern Hemisphere conifers (except Pinaceae): Cupressaceae, Taxodiaceae | Trees with VAM (see below) |
| Early Jurassic (190 Myr) to Present | Gnetales: Ephedra, Gnetum, Welwitschia | VAM or ECM (Gnetum) (see below) |
| Early Cretaceous? (>120 Myr) to Present | Conifers in Pinaceae: Larix, Picea, Pinus, Tsuga, etc. | ECM trees with heterorhizic roots (see below) |
| Early Cretaceous (~120 Myr) to Present | Angiosperms | VAM, NM or other mycorrhiza type (see section ? below) |
| Cretaceous (100 Myr) to Present | Fagales: Betulaceae, Casuarinaceae, Fagaceae, Juglandaceae, Myricaceae, Nothofagaceae | Single lineage of ECM trees or shrubs with heterorhizic roots (some VAM also) (see Section 5) |
| Late Cretaceous, Oligocene, or Eocene to present (90-30 Myr) | Fabaceae (Caesalpiniaceae, Mimosaceae), Myrtaceae, Salicaceae, Tiliaceae, etc. | Several separate ECM lineages (see Section 5) |
| Cretaceous (100 Myr) to Present | Orchidaceae | Orchid mycorrhizas. Orchids age estimate based on biogeography and phylogenetics (Chase 2001) |
| Cretaceous (100 Myr) to Present | Mainly NM families: Proteaceae, Cyperaceae, Restionaceae, etc. | Oldest known fossils of plants likely to have true NM roots |
| Late Cretaceous (80 Myr) | Ericaceae | Oldest known fossils (Nixon & Crepet 1993) likely to have had ericoid mycorrhizas (Cullings, 1996) |
This table was originally published in Brundrett (2002). The mycorrhizal status of lineages is primarily derived from observation of living descendants with some fossil evidence. Data on plant lineages and fossil histories are from Stewart & Rothwell (1993), Taylor & Taylor (1993), Kenrick & Crane (1997), Wing & Boucher (1998), Hill et al. (1999), Renzaglia et al. (2000) and Barrett & Wills (2001). All dates are approximate. Abbreviations: VAM = arbuscular mycorrhizal, ECM = ectomycorrhizal, NM = nonmycorrhizal.
Table 2. The early history of fungi in the Glomeromycota thought to form vesicular-arbuscular mycorrhizas based on fossil evidence and DNA sequence data.
| Period | Evidence |
| Cambrian? >500 Myr | Fungi probably colonised land long before plants, but left scant early fossil evidence (Taylor & Osborn 1996) |
| Proterozoic? >570Myr | DNA sequences show Glomeromycotan fungi are the monophyletic sister group to the higher fungi (Schüßler et al. 2001, Tehler et al. 2000) |
| 400-500 Myr | Approximate origin of Glomeromycota according to a DNA molecular clock estimates (Berbee & Taylor 1993, Simon et al. 1993) |
| Ordovician (460 Myr) | Fossil spores identified as Glomeromycotan fungi (Pirozynski & Dalpé 1989, Redecker et al. 2000a) |
| Devonian (400 Myr) | Fossil spores identifiable as Scutellospora, a genus within the Glomeromycota (Dotzler et al. 2008) |
| Devonian (400 Myr) to present | Many reports of fossil spores likely to be Glomeromycotan (Pirozynski & Dalpé 1989) |
| Unknown to present | Geosiphon – a soil crust fungus with endosymbiotic cyanobacteria that rRNA sequence data suggests is a primitive Glomeromycotan fungus (Schüßler & Kluge, 2000) |
C. Mycorrhizal Status of Primitive Plants
1. Bryophytes
Bryophytes might be expected to be nonmycorrhizal since they lack roots - the organ which is normally required to host these associations and have a dominant gametophyte stage. However, some of these primitive plants have associations with glomeromycete fungi, or other fungi in their thallus. Only a brief summary provided here refer to recent reviews for more information: Read et al. (2000), Nebel et al. (2004), Kottke & Nebel (2005) and Ligrone et al. (2007).
- Liverworts (Hepatophyta: 15 orders or families)
- Associations with Glomeromycotan fungi in a thallus considered to be like VAM. Examples include Treubia sp. which has a coiling associations without arbuscules (Duckett et al. 2006), as well as Conocephalum, Haplomitrium and Pellia spp. which have arbuscules (Turnau et al. 1999, Carafa et al. 2003).
- Liverworts (Hepatophyta: 11 families or orders)
- Liverworts where mycorrhizas were not seen in samples examined include members of the families Metzgeriaceae, Pleuroziaceae, Sphaerocarpales, Ricciaceae and some Aneuraceae (Riccardia spp.) (Nebel et al. 2004, Ligrone et al. 2007).
- Liverworts (Hepatophyta: Makinoaceae, Metzgeriaceae)
- Mycorrhiza-like associations with basidiomycetes that include members of the Rhizoctonia alliance (Kottke et al. 2003, Duckett et al. 2006).
- Mosses (Bryophyta)
- Saprophytic activity of both VAM and ECM fungi occurs in senescent moss tissue (see review by Davey & Currah 2006). Zhang & Guo (2007) observed hyphae and vesicles of Glomeromycotan fungi in a wide diversity of moss species.
- Hornworts (Anthocerotophyta)
- Anthoceros and Phaeoceras have associations by Glomeromycotan fungi with arbuscules (Ligrone 1988, Schüßler 2000).
2. Fern Allies
Selaginella, Isoetes and Lycopodium are primitive plants that belong to a separate clade between the ferns and hornworts (bryophytes) (Pryer et al. 2004, Qui et al. 2007). Living relatives of some of the earliest land plants typically have VAM associations, but some of these relationships are hard to decipher, especially in myco-heterotrophic gametophytes (Read et al. 2000, Brundrett 2002).
- Huperzia, Lycopodium, Phylloglossum (Huperziaceae, Lycopodiaceae)
- Lycopodiaceae typically have VAM in sporophytes and myco-heterotrophic VAM in gametophytes, but some samples are nonmycorrhizal (Boullard 1969, Laferriére & Koske 1981, Gemma et al. 1992, Schmid & Oberwinkler 1993, Gemma & Koske 1995, Johnston & Ryan 2000, Winther & Friedman 2008).
- Selaginella (Selaginellaceae)
- Most species examined had roots with VAM (Maeda 1954, Laferriére & Koske 1981, Harley & Harley 1987, Väre et al. 1997, Gemma et al. 1992, Zhao 2000).
- Isoetes (Isoetaceae)
- These submerged aquatic plants often contain VAM associations, even when growing at considerable depths (Laferriére & Koske 1981, Clayton & Bagyaraj 1984, Farmer 1985, Ragupathy et al. 1990, Khan & Belik 1995, Sharma 1998*, Wigand et al. 1998). *Sharma (1998) shows an unidentified association likely to be VAM.

Lycopodium sp. |

Selaginella sp. |

Ophioglossum lusitanicum |
3. Primitive Ferns
Phylogenetic analyses based on gene sequence data have shown that the “fern allies” such as Equisetum and Psilotum are in a monophyletic clade with other ferns (Pryer et al. 2004, Qui et al. 2007). Subterranean gametophytes of primitive ferns have myco-heterotrophic VAM associations with hyphal coils, but no arbuscules (Peterson et al. 1981, Schmidt & Oberwinkler 1994, Duckett & Ligrone 2005).
- Psilotum, Tmesipteris (Psilotaceae)
- Coiling VAM in subterranean gametophyte (Peterson et al. 1981, Gemma et al. 1992, Duckett & Ligrone 2005). Often NM when growing as an epiphyte or in potting mix - Gemma & Koske 1995, Brundrett unpublished. Sporophytes and gametophytes have VAM with hyphal coils that lack arbuscules in their rhizomes (Gemma et al. 1992).
- Botrychium, Helminthostachys, Ophioglossum (Ophioglossaceae)
- Botrychium and Ophioglossum sporophytes have VAM and subterranean gametophytes of these plants have myco-heterotrophic VAM (Maeda 1954, Berch & Kendrick 1982, Gemma et al. 1992, Schmidt & Oberwinkler 1996, Zhao 2000, Winther & Friedman 2007).
- Equisetum (Equisetaceae)
- Equisetum sporophytes can have VAM or NM roots and probably have facultative associations, as some individuals are NM (Laferriére & Koske 1981, Koske et al. 1985, Khan & Belik 1995, Berch & Kendrick 1982, Koske et al. 1985, Zhao 2000). In one study 11 of 12 samples had VAM (Dhillion 1993), while another found VAM in 21 of 23 samples (Koske et al. 1985).
 |
 |
Huperzia (Lycopodium) phlegaria (Lycopodiaceae) - Tassel fern, a Lycopod. |
4. Ferns
Comprehensive studies of ferns listed below in different regions of the world found the majority to be mycorrhizal with VAM associations. However, most ferns have fine roots with long root hairs (shown below), suggesting they have facultative mycorrhizas. More advanced Leptosporangiate ferns are considered to be less dependent on mycorrhizas than more primitive ferns (Boullard 1979, Gemma et al. 1992, Zhao 2000). Photosynthetic gametophytes of ferns are usually not mycorrhizal (Cooper 1976, Harley & Harley 1987), but Pellaea and Adiantum had VAM in their gametophytes (Turnau et al. 2005). Substrates strongly influence mycorrhizal status of ferns, since aquatic ferns, such as Azolla, Marsilea and Salvinia, and epiphytes are less likely to be mycorrhizal than terrestrial species (Maeda 1954, Gemma et al. 1992, Nadarajah & Nawawi 1993, Lehnert et al. 2009).
Mycorrhizal Surveys of Ferns and Fern Allies
- VAM in 80 out of 85 species from Japan (aquatic ferns NM) (Maeda 1954).
- VAM in 94 of 101 species in 21 families from New Zealand by Cooper (1976).
- VAM in 21 out of 35 species from Europe in data summarised by Harley & Harley (1987).
- VAM in 17 out of 33 species in Canada (Berch & Kendrick 1982).
- VAM in 66 out of 89 species (83% of terrestrial vs. 55% of epiphytic ferns) in Hawaii by Gemma et al. (1992).
- VAM in 13 out of 22 epiphytic species in Hawaii by Gemma & Koske (1995).
- 20 species of epiphytic ferns in Malaysia were not mycorrhizal, but many of the same species had VAM in soil (Nadarajah & Nawawi 1993).
- VAM in 88 out of 256 species in Yunan Province, China by Zhao (2000).
- VAM in 31 out of 34 species sampled in southern China by Zhang et al. (2004).
- VAM in 19 out of 85 species in sothern Ecuador by Lehnert et al. (2009).
4. Gymnosperms
The gymnosperms are the next major group to evolve after the ferns and gave rise to the angiosperms (Stewart & Rothwell 1993, Qui et al. 2007). There are two major mycorrhizal strategies of gymnosperms: VAM and ECM that follow major clades. The genus Gnetum and genera in the Pinaceae have ECM roots, while all the rest have VAM. Gymnosperms are classified into groups below from the most to least primitive following molecular phylogenies (Pryer et al. 2004, Qui et al. 2007).
- Ceratozamia, Cycas, Dioone, Lepidozamia, Macrozamia, Zamia - Cycads (Cycadaceae, Zamiaceae, Stangeriaceae)
- VAM occur in cycad roots sampled from India, Florida USA, Australia, etc. (Brundrett & Abbott 1991, Reddell et al. 1996, Muthukumar & Udaiyan 2002, Fisher & Vovides 2004). See the Cycad Pages for information on cycad identification, ecology, propagation, Nitrogen fixing associations, etc.
- Ginkgo biloba - Maidenhair Tree (Ginkgoaceae)
- The only living member of this clade is a tree with VAM (Maeda 1954, Bonfante-Fasolo & Fontana 1985).
- Ephedra (Ephedraceae)
- VAM hosts (Selivanov & Eleusenova 1974, Bethlenfalvay et al. 1984, Titus et al. 2002).
- Gnetum (Gnetaceae)
- Tropical climbers with ECM association, as illustrated below (Fassi 1957, St. John 1980, Ongene & Kuyper 2001).
- Welwitschia mirabilis (Welwitschiaceae)
- One remaining species in family. VAM - Jacobson et al. (1993).
- Abies, Cathaya, Cedrus, Keteleeria, Larix, Picea, Pinus, Pseudolarix, Pseudotsuga, Tsuga (Pinaceae)
- A great many studies of plants in this family (that includes pine trees and other conifers important to forestry) have shown them to be ECM hosts, as summarised by Harley & Harley (1987) and Brundrett et al. (1990). These trees and their associated fungi dominate boreal forests in the Northern Hemisphere. Roots of these trees also contain hyphae of VAM fungi, presumably as non-functional (endophytic) associations (Cázares & Trappe 1993, Wagg et al. 2008).
- Agathis, Araucaria, Phyllocladus, Podocarpus, Wollemia, etc. (Araucariaceae, Podocarpaceae)
- These conifers were once dominant in forests worldwide, but now confined to relatively cool and wet areas in the Southern Hemisphere. They consistently have VAM associations in beaded roots (Baylis et al. 1963, Bevege, 1968, Johnson 1977, Allsopp & Stock 1993, Reddell et al. 1996, McGee et al. 1999, Hurst et al. 2002, Russell et al. 2002). Parasitaxus usta is an unusual parasitic plant in the Podocarpaceae from New Caledonia that apparently has both mycorrhizal and physical connections with its host (Field & Brodribb 2005)
- Actinostrobus, Austrocedrus, Callitris, Calocedrus, Cephalotaxus, Chamaecyparis, Cryptomeria, Cunninghammia, Cupressus, Juniperus, Metasequoia, Sequoia, Sequoiadendron, Taxodium, Taxus, Thuja, Thujopsis, Torrea, Widdringtonia (Cupressaceae)
- These conifers include the largest and some of the longest-lived trees in the world (Farjon 2005). The genera listed above have VAM associations (Maeda 1954, Kough et al. 1985, Newman & Reddell 1987, Harley & Harley 1987, Fontenla et al. 1989, Brundrett et al. 1990, Allsopp & Stock 1993, Cantelmo & Ehrenfeld 1999, Wubet et al. 2003). VAM associations also occur in fossil Metasequoia roots (Stockey et al. 2001). Older reports of ECM in this group are probably incorrect (see Reinsvold & Reeves 1986 for examples). The current definition of Cupressaceae includes the Cephalotaxaceae, Taxaceae, etc. (Farjon 2005).
- Sciadopitis (Sciadopityaceae)
- Roots of plants in this family, outgroup to the Cupressaceae, also have VAM (Maeda 1954).
 |

|
Encephalartos ferox a Cycad (Zamiaceae) with female cones. |
Agathis robusta (Araucariaceae) - a southern hemisphere conifer. |
| Conifers with VAM | Conifers with ECM |
 |
 |
Callitris columellaris (Cupressaceae) - Cyperus Pine (Western Australia). |
Picea glauca (Pinaceae) - White Spruce (Ontario, Canada). |
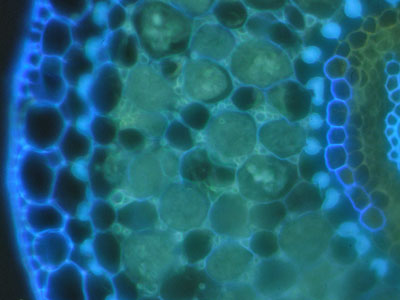 |
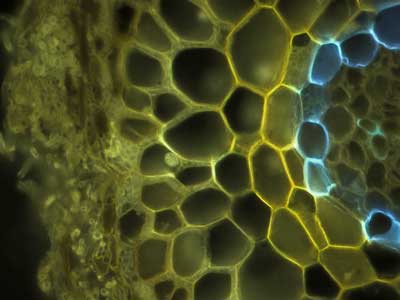 |
Thuja occidentalis (White Cedar, Cupressaceae) autofluorescence image of VAM in root cross section (Ontario, Canada). |
Larix laracina (Larch, Pinaceae) autofluorescence image of ECM cross section (Ontario, Canada). |
 |
 |
|
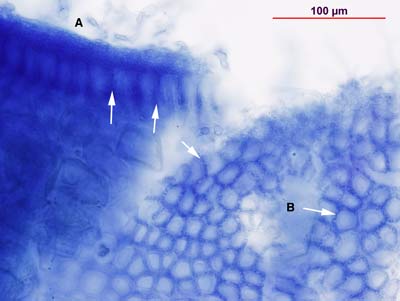
Squashed cleared and stained root (left) and section (right) of Gnetum sp. short root showing Hartig net in (A) side and (B) top views. Arrows point to Hartig net hyphae. This association has a thick mantle (M) and an epidermal Hartig net (H) which differs from the cortical Hartig net of ECM conifers. | |
D. Mycorrhizas of Flowering Plants
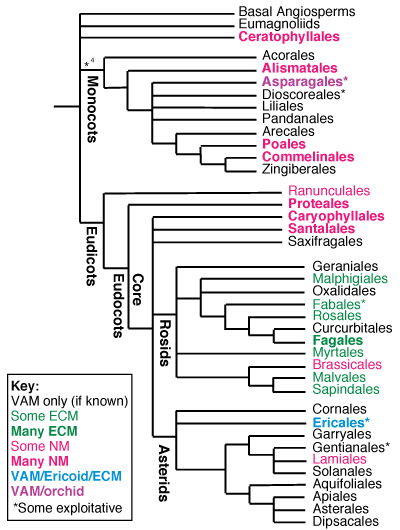
The strongest evidence that VAM is the ancestral condition for angiosperms is provided its near- ubiquitous occurrence in these plants today (Baylis, 1975; Trappe, 1987; Newman & Reddell, 1987). Trappe (1987) compiled data for 6507 angiosperm species, of which 67% had VAM (including 12% considered to be facultative), 15% had another association type and 18% were NM. A more recent survey by Wang & Qui (2006) of 2964 species found very similar results (85% of species mycorrhizal). Other summaries of the UK flora (Harley & Harley, 1987), Hawaiian angiosperms (83% mycorrhizal - Koske et al., 1992) and Australian plants (Section 8) of plants from natural ecosystems have provided similar results.
A brief summary of mycorrhizal lineages in the angiosperms is provided below primarily based on data from my review (Brundrett 2002). These lineages are shown in the Figures below and the dates when they diverged are summarised in the mycorrhizal history table above. Comprehensive lists of ECM hosts, NM plants and Australian plants are provided in Sections 5, 6 and 8 of this site.
- Ectomycorrhizal Associations in the Fagales
- It is probable that the origin of ECM in the Fagales occurs in the Cretaceous after the Pinaceae already had these associations. Information on the The Fagales lineage (Betulaceae, Casuarinaceae, Juglandaceae, Nothofagaceae and Fagaceae) is summarised in Section 5.
- ECM in other families
- Other angiosperms that have independently evolved the capacity to host ECM include some members of the Ericaceae and 11 families in 6 orders of the rosids (see Section 5). These clades include many families of VAM plants, so have more recently become ECM hosts. The Dipterocarpaceae, Cistaceae and Sarcolaenaceae are closely related ECM families in the Malvales that share an common ancestor (Ducousso et al. 2004). Several isolated lineages of ECM plants occur in otherwise NM families: the sedges (Kobresia: Cyperaceae) and several genera in the Caryophyllales (Neea, Pisonia: Nyctaginaceae) (see Brundrett 2002, Bruns & Shefferson 2004).
- Mycorrhizas in the Ericaceae
- Members of the Ericaceae have complex evolutionary trends, starting from a VAM ancestor, progressing to ECM, then to arbutoid ECM and culminating in ericoid mycorrhizas, or exploitative ECM in myco-heterotrophs like Monotropa.
- Orchid Mycorrhizas (Orchidaceae)
- orchids are a single consistent lineage with orchid mycorrhizas.
- Nonmycorrhizal (NM) Plants
- There are at least 10 lineages of NM plants in the angiosperms, but most also contain VAM plants. As explained in Section 6, NM lineages include many aquatic plants, parasitic plants, insectivorous plants that often occur in harsh habitats, such as wet, saline, or arid soils.
- Myco-heterotrophic Plants with Exploitative Mycorrhizas
- Plants with exploitative mycorrhizas occur in many separate lineages (Molvray et al. 2000). These include subterranean gametophytes of primitive plants, as well as angiosperms with exploitative mycorrhizas in the dicotyledon's (Diapensiaceae, Ericaceae, Gentianaceae, Polygalaceae) and the monocots (Burmanniaceae, Corsicaceae, Iridaceae, Orchidaceae, Petrosaviaceae, Triuridaceae). Knowledge of myco-heterotrophic mycorrhizas is summarised by Furman & Trappe (1971), Leake (1994), Brundrett (2002), Bidartondo (2005). An online list of myco-heterotrophic plants is provided by Dan Nickrent.
Comparing data on the occurrence of mycorrhizas in Angiosperm families with phylogenies based on multiple gene sequence data (Brundrett 2002)
Hover mouse over diagram for animated version.
Evolutionary lineages of plants with different types of mycorrhizas or loss of mycorrhizal dependency (Brundrett 2002)
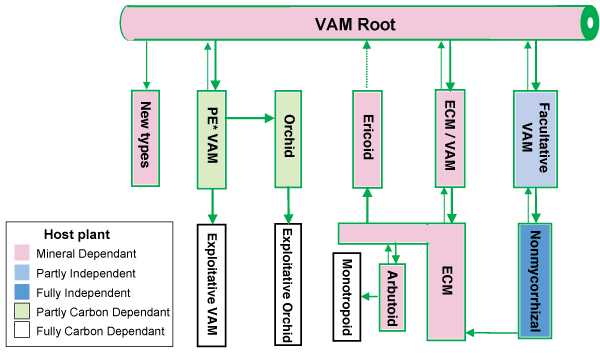
|
Hover mouse to see approximate numbers of new lineages.
- Boxes and arrows are not to scale
- PE = partially exploitative
- See Brundrett 2002 for more information on these evolutionary trends
E. Evolution of Roots
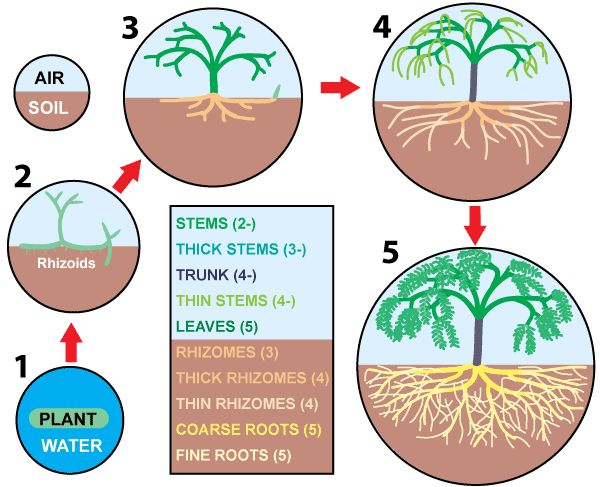
Hypothetical events in the evolution of roots are summarised in the diagram below. As plants colonised the land they would have faced powerful selection pressure to increase the surface area of their absorptive systems in soil in parallel with increases in their photosynthetic organs (fine branches and leaves). The evolution of roots most likely started by specialisation of underground stems (rhizomes) as shown in the diagram below, but there is little fossil evidence of intermediate stages in this process.
Roots continued to progressively evolve into a hierarchy of structures (branch orders), with the finest elements becoming progressively thinner and more diffuse, culminating in the very complex roots in angiosperms with NM cluster roots. Fine roots tend to be short-lived, while relatively coarse roots form a more-permanent network used for mechanical support, production of fine roots and transport. Structural evidence that roots evolved with the need to provide habitats for mycorrhizal fungi as a primary function is summarised Section 3.
Hypothetical Stages in Root Evolution
- Aquatic plants without roots or leaves colonise land
- Early land plants with upright branches to intercept sunlight and horizontal stems (rhizomes) for propagation.
- Complex shoot and rhizome branching allows more effective use of resources and competition with other plants.
- Dimorphic subterranean rhizome systems evolve in response to allow more optimal nutrient and water uptake, mycorrhiza formation, and mechanical support. This occurs in parallel with the evolution of specialised aerial branches that are more efficient at photosynthesis.
- Some rhizomes become thinner and longer to increase contact with the soil and absorptive capacity, eventually becoming specialised as roots. These host mycorrhizal associations, grow faster and have a relatively short lifespan. In contrast, the thick rhizomes evolve protective features to limit permeability and facilitate long-term survival in soil.
F. Summary and Conclusions
- The >400 Myr coevoluton of mycorrhizal fungi and roots of land plants is summarised using data from paleobotanical and morphological studies and DNA-based phylogenies.
- The first bryophyte-like land plants, in the early Devonian (400 million years ago), had fungal associations resembling arbuscular mycorrhizas (VAM) even before roots evolved.
- Fossil evidence shows that plants have had VAM associations (or something very similar) since they first colonised land in the early Paleozoic, and these associations seem to have changed very little in the hundreds of millions of years since roots evolved.
- Mycorrhizal evolution in the first land plants is considered to have progressed from endophytic towards balanced associations as partners became interdependent due to the exchange of limiting energy and nutrient resources (see Brundrett 2002).
- Most mycorrhizas are mutualistic, but in some cases the trend for increasing plant control of fungi culminates in the exploitative mycorrhizas of achlorophyllous, myco-heterotrophic plants.
- Ectomycorrhizal, ericoid and orchid mycorrhizas, as well as nonmycorrhizal roots, evolved during the period of rapid angiosperm radiation in the Cretaceous.
- It is hypothesised that roots gradually evolved from rhizomes to provide more suitable habitats for mycorrhizal fungi and balance the increased needs for water and nutrients of large plants with leaves.
- As discussed in Sections 3 and 7, selection pressures have caused the morphological divergence of roots with different types of mycorrhizas (e.g. heterorhizy in ECM roots). Root cortex thickness and exodermis suberisation are greatest in obligately mycorrhizal plants, while nonmycorrhizal plants tend to have fine roots, with more roots hairs and relatively advanced chemical defences.
- Major coevolutionary trends and the relative success of plants with different root types are discussed further in the Tansley Review.
© Mark Brundrett 2002, 2008