MYCORRHIZAL ASSOCIATIONS: The Web Resource
Section 5. ECTOMYCORRHIZAS
A. Introduction
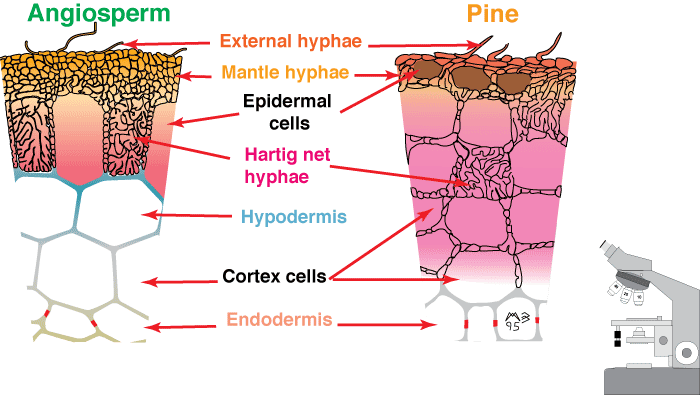
Ectomycorrhizal associations (abbreviated as ECM, or EM) are mutualistic associations between higher fungi and Gymnosperms or Angiosperms in the plant families listed here. As illustrated below, ECM associations consist of a soil mycelium system, linking mycorrhizal roots and storage or reproductive structures. Ectomycorrhizal roots (formerly known as ectotrophic or sheathing mycorrhizas) are characterised by the presence of a mantle and Hartig net.
Ectomycorrhizal associations are formed predominantly on the fine root tips of the host, which are unevenly distributed throughout the soil profile, being more abundant in topsoil layers containing humus, than in underlying layers of mineral soil (Meyer 1973, Harvey et al. 1976). ECM fungi can make a significant contribution to the biomass of forest ecosystems (Marks et al. 1968, Vogt et al. 1981, Hunt & Fogel 1983). An brief introduction to ECM fungi is provided in Section 9.
| Topic | Major Sources |
| Images | Malajczuk et al. 1982, Piché et al. 1983, Brundrett et al. 1990, Brundrett et al. 1996 |
| Diagrams and definitions | Brundrett et al. 1996 |
| Host plants | Brundrett 2009 |
B. Looking at ECM
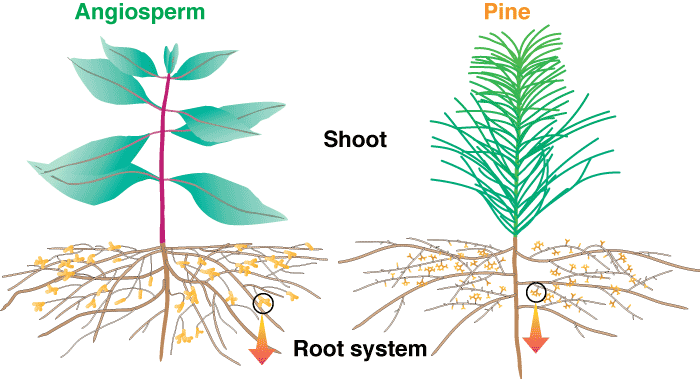
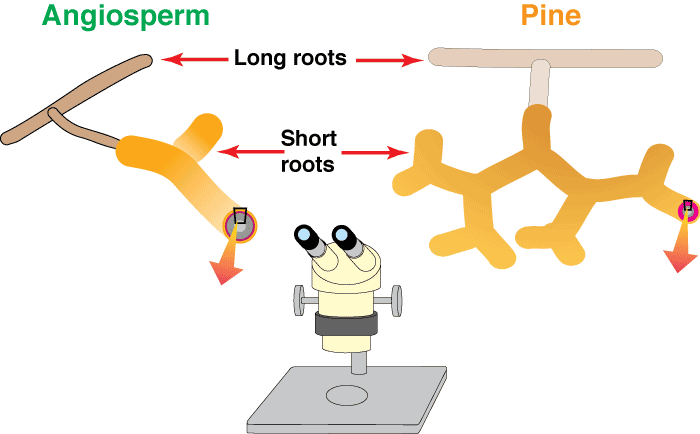
These diagrams show increasingly magnified views of typical angiosperm and pine mycorrhizal root systems with characteristically branched short roots and ectomycorrhizal structures. Information on the methods required to see mycorrhizal structures is provided in Section 10.
Diagrams on this page are also available as pdf files to download.
Level 1. Whole Plants
 |
|
|
|
Level 2. The Dissecting Microscope
 |
|
|
Level 3. The Compound Microscope
 |
|
|
|
C. Structures and Developmental Stages
Ectomycorrhizas form by synchronised growth by host roots and compatible fungi when environmental conditions are favourable. The sequence of events that results in ECM formation has been described in many studies (Chilvers & Gust 1982, Kottke & Oberwinkler 1986, Massicotte et al. 1987, Peterson et al. 2004). These events are summarised below.
1. Root Systems
Most ECM roots have a modified lateral root branching pattern. This pattern, which is called heterorhizy, consists of short mycorrhizal lateral roots (called short roots) supported by a network of long roots. The long and short roots in heterorhizic root systems are fundamentally similar in structure, but short roots normally grow much more slowly than long roots (Wilcox 1964, Kubíková 1967). The restricted growth of short roots may be necessary to allow ECM fungi time to form an association, since these fungi have difficulty colonising more rapidly growing roots (Chilvers & Gust 1982). Thus, trees with ECM would require slow growth of some of their lateral roots, and in time, this process would result in the evolution of separate, genetically distinct long and short roots. The long roots of many species rapidly develop a periderm, which prevents them from forming mycorrhizas.
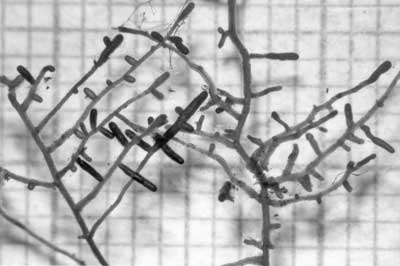 |
Example of ECM short roots (arrows) of birch (Betula alleghaniensis), an angiosperm tree. These structures are explained below.
|
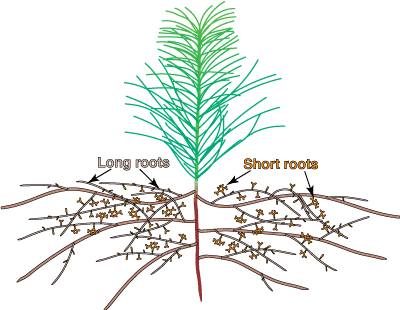 |
Diagram of the roots system of a pine seedling to illustrate long and short roots. The mycorrhizal short roots are thicker than other laterals of the same order due to the mantle and Hartig net. 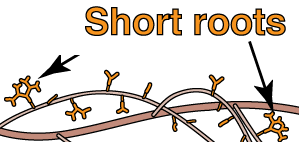 |
2. Soil Hyphae
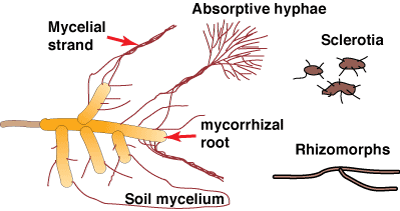 |
Mycorrhizal fungi produce a hyphal network in soil consisting of individual strands of hyphae and/or relatively undifferentiated bundles of hyphae called mycelial strands, or rhizomorphs with specialised conducting hyphae (Agerer 1995). Sclerotia, which are larger, resistant storage structures, may also be produced. Soil hyphae function by acquiring nutrients re-allocating resources for fungus reproduction or mycorrhizal exchange and by functioning as propagules for survival and spread of the fungus (Ogawa 1985, Agerer 1995, Unestam & Sun 1995). |
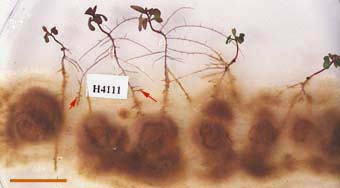 |
Hyphae of a Pisolithus species growing on a Petri dish forming mycorrhizas (arrows) with Eucalyptus grandis seedlings in sterile culture (see Burgess et al. 1994).
|
 |
ECM short roots (S) of Eucalyptus globulus with a Cenococcum-like mycorrhiza that has relatively thick black radiating external hyphae (arrow).
|
3. Root Contact and Hyphal Proliferation
Hyphae contact, recognise and adhere to root epidermal cells near the apex of young, actively growing, high-order, lateral root. These laterals are called short roots because they normally have restricted growth. Early stages in the establishment of ECM associations are illustrated by Scanning Electron Microscope (SEM) images of the development of ECM of Pinus strobus by Piché et al. (1983). These show attached hyphae on the root surface 1-2 days after first contact with the root and the mantle and Hartig net after 2-4 days.
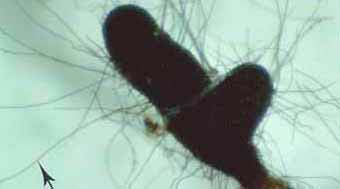
 |
Early stage of colonisation of pine short root by Pisolithus tinctorius. Hyphae (arrows) are starting to grow near the apex of a short root (A).
|
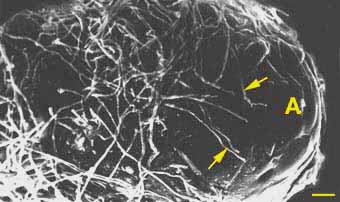
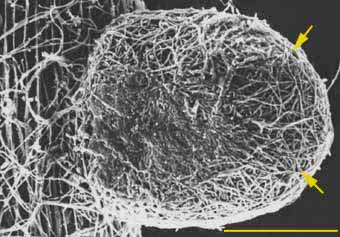 |
SEM image of a later stage of pine root colonisation by Pisolithus tinctorius. Mantle hyphae have formed a dense covering on the root surface (arrows).
|
4. Mycorrhizal Roots
After ECM associations are established, mycorrhizal short roots often continue to grow by elongation and branching. Conifer roots with ECM have dichotomous branching patterns, while angiosperms have sympodial branching. The size, colour, texture and branching patterns of ECM roots vary with different host-fungus combinations, which are called morphotypes. The images below, from a host-fungus compatibility study (Malajczuk et al. 1982), illustrate morphotype variation and some eucalyptus ECM morphotypes are illustrated in Section 9.
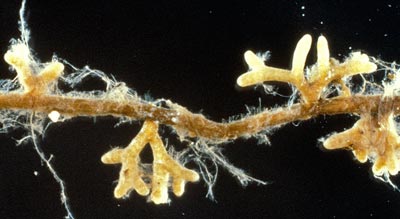 |
Ectomycorrhizal association synthesised under sterile conditions between Pinus radiata and Suillus brevipes. These dichotomously branched mycorrhizal short roots increase in age from left to right.
|
 |
Pinus radiata and Amanita muscaria ECM synthesised under sterile conditions. This association has highly branched short roots with many root tips coated in white mycelium.
|
 |
Eucalyptus maculata and Astraeus pteridis association synthesised under sterile conditions with relatively unbranched ECM and attached mycelial strands.
|
5. The Hartig Net
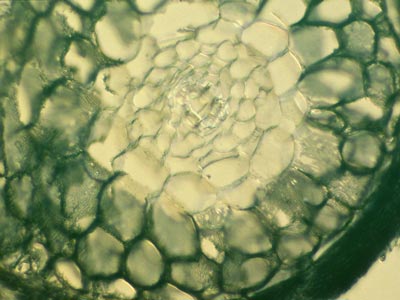
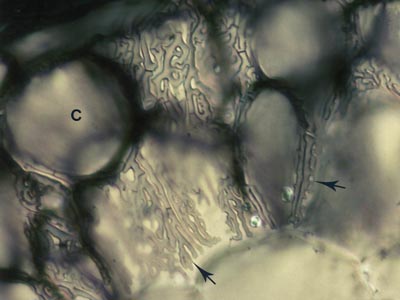
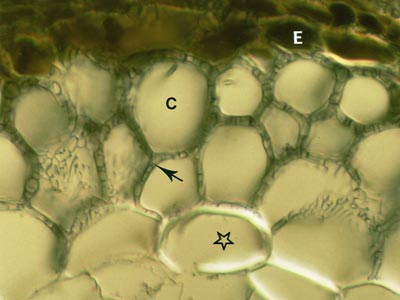
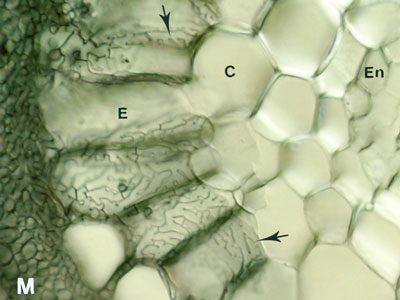
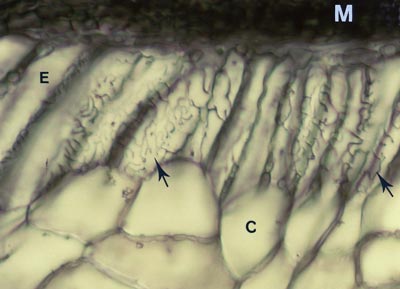
Hyphae penetrate between host cells and branch to form a labyrinthine structure called the Hartig net. Host responses may include polyphenol production in cells and the deposition of secondary metabolites in walls (Weiss et al. 1997, Ling-Lee et al. 1977). Angiosperms with ECM, such as Eucalyptus, Betula, Populus, Fagus, Shorea, etc., usually have a one cell layer Hartig net confined to the epidermis (Alexander & Högberg 1986, Peterson et al. 2004). This is the epidermal category of ECM (Brundrett 2004). This contrasts with the typical situation in gymnosperms such as Pinus, where Hartig net hyphae extend deep into the cortex (Kottke & Oberwinkler 1986, Brundrett et al. 1990), in the cortical category of ECM (Brundrett 2004). Structural characteristics of cells in the hypodermal layer are thought to restrict Hartig net hyphae to the epidermis in most angiosperms, but not in gymnosperms (Ling-Lee et al. 1977, Brundrett et al. 1990). Typical examples of ECM roots are shown below.
The active mycorrhizal zone occurs several mm behind the root tip (as a result of the time required for mycorrhizal formation), but Hartig net hyphae senesce in older regions further from the root tip (Massicotte et al. 1987). Consequently, ECM associations are dependant on root growth. The mantle in older roots generally persists long associations become inactive, presumably to function as a storage structure.
The images below are hand sections of Canadian forest trees cleared and stained with Chlorazol black E and viewed with interference contrast microscopy (Brundrett et al. 1990). |
Rollover shows close-up of Hartig net
6. Reproduction
The hyphal network that interconnects ECM fungi in soils is also responsible for reproduction. Fruit bodies grow from primordia at times of the year when environmental conditions are favourable. Some fungi will fruit under mycorrhizal plants growing in pots, as shown below.
 |
Fruit bodies of an ECM fungus (Laccaria sp.) under a Eucalyptus globulus seedling inoculated with fungal mycelia.
|
D. Host Plants
Trees with ECM associations are dominant in coniferous forests, in cold boreal or alpine regions, and many of the broad-leaved forests in temperate or mediterranean regions, but they also occur in some tropical or subtropical savanna or rain forests habitats (Meyer 1973, Alexander 1989, Brundrett 1991). Families and genera of plants with ECM are listed in the Table below.
At least 10 lineages of Angiosperms have independently evolved the capacity to host ECM (Brundrett 2002, Bruns & Shefferson 2004). The majority of hosts are trees, or shrubs (see Table), but associations are formed by a few herbaceous plants, including Kobresia (Cyperaceae) and Polygonum (Polygonaceae) species found in alpine/arctic regions (Kohn & Stasovski 1990, Massicotte et al. 1998). Some Australian herbaceous plants are reported to have ECM (Kope & Warcup 1986, McGee 1988), but this is controversial due to the lack of Hartig net in these associations.
Families and genera with confirmed ectomycorrhizal associations (see Brundrett 2009 for updated list)
| Family | Genera | References |
| GYMNOSPERMS | ||
| Gnetaceae | Gnetum | Fassi 1957, St. John 1980, Onguene & Kuyper 2001. See photos in Section on evolution. |
| Pinaceae | Abies, Cathaya, Cedrus, Keteleeria, Larix, Picea, Pinus, Pseudolarix, Pseudotsuga, Tsuga | Malloch & Malloch 1981, Harley & Harley 1987, Brundrett et al. 1990 |
| ANGIOSPERMS | ||
| Betulaceae (inc. Corylaceae) | Alnus, Betula, Carpinus, Corylus, Ostrya, Ostryopsis | Rose 1980, Harley & Harley 1987, Brundrett et al. 1990 ] |
| Casuarinaceae | Allocasuarina, Casuarina | Theodorou & Reddell 1991 |
| Cistaceae | Cistus, Fumana, Helianthemum, Hudsonia, Lechea, Tuberaria | Giovannetti & Fontana 1982, Malloch & Thorn 1985, Wang & Qui 2006, Comandini et al. 2006 |
| Dipterocarpaceae | Anisoptera, Dipterocarpus, Hopea, Marquesia, Monotes, Pakaraimaea, Shorea, Vateria, Vateriopsis, Vatica | Alexander & Högberg 1986, Högberg & Piearce 1986, Smits 1992, Moyersoen et al. 2001, Moyersoen 2006, Tedersoo et al. 2007 |
| Ericaceae* *Many other genera with ericoid mycorrhizas |
Arbutoid category of ECM (and ECM): Arbutus, Arctostaphylos, Cassiope, Chimaphila, Comarostaphylis, Gaultheria, Kalmia, Leucothoe, Pyrola
Monotropoid category of ECM: Monotropa, Pterospora, Sarcodes, etc. |
Arbutoid: Largent et al. 1980, Molina & Trappe 1982, Massicotte et al. 1998, Massicotte et al. 2005a, Osmundson et al. 2007.
Monotropoid:Robertson & Robertson 1982, Castellano & Trappe 1985, Bidartondo et al. 2000. Gaultheria and Kalmia also have ericoid mycorrhizas - Massicotte et al. 2005b. |
| Fabaceae (Leguminosae / Fabales) | ||
| Caesalpinioideae (Caesalpiniaceae) | Afzelia, Anthonotha, Aphanocalyx, Berlinia, Brachystegia, Cryptosepalum, Dicymbe, Didelotia, Eperua, Gilbertiodendron, Gleditsia, Intsia, Isoberlinia, Julbernardia, Microberlinia, Monopetalanthus, Paraberlinia, Paramacrolobium, Pellegriniodendron, Tetraberlinia, Toubaouate | see Alexander 1989 for summary. Other references - Fassi 1962, Alexander & Högberg 1986, Bakarr & Janos 1996, Thoen & Ba 1989, Frioni et al. 1999, Onguene & Kuyper 2001, Henkel et al. 2002, Tedersoo et al. 2007. |
| Papiloinoideae (Papilionaceae) | Aldinia, Gastrolobium, Gompholobium, Jacksonia, Lonchocarpus, Mirbelia, Oxylobium, Pericopsis | See Australian peas. Aldinia in Guyana - Henkel et al. 2002, Pericopsis in Uruguay - Frioni et al. 1999 |
| Mimosoideae (Mimosaceae) | Acacia, Calliandra | Australian acacias, Frioni et al. 1999 |
| Fagaceae | Castanea, Castanopsis, Fagus, Lithocarpus, Quercus | Harley & Harley 1987, Brundrett et al. 1990, Haug et al. 1991, Jurgensen et al. 1997, Moyersoen et al. 2001. Chrysolepsis and Trigonobalanus are probably also ECM. |
| Junglandaceae | Carya, Engelhardtia | Harley & Harley 1987, Brundrett et al. 1990, Haug et al. 1991, Jurgensen et al. 1997 |
| Melastomataceae | ECM (ectendomycorrhizal) association with ascomycete (Hymenoscyphus), VAM also: Graffenrieda | Haug et al. 2004 |
| Meliaceae | Owenia | Brundrett et al. 1995 |
| Myrtaceae | Allosyncarpia, Agonis, Angophora, Baeckea, Eucalyptus, Leptospermum, Melaleuca, Tristania, Tristaniopsis | see Australian Myrtaceae |
| Nothofagaceae (Fagaceae) |
Nothofagus | Warcup 1980, McKenzie et al. 2000, Perrier et al. 2006 |
| Nyctaginaceae | Guapira, Neea, Pisonia | St John 1980, Ashford & Allaway 1985, Moyersoen 1993, Chambers et al. 2005, Haug et al. 2005. |
| Phyllanthaceae (Euphorbiaceae) | Uapaca*, Ampera, Poranthera (*formerly in Uapaceaceae) |
Alexander & Högberg 1986, Onguene & Kuyper 2001, Ramanankierana et al. 2007. |
| Polygonaceae | Polygonum (arctic and alpine herbs), Coccoloba (tropical tree) |
Polygonum - Kohn & Stasovski 1990, Massicotte et al. 1998, Mühlmann et al. 2008. Coccoloba - Koske et al. 1992, Béreau et al. 1996. |
| Rhamnaceae | Cryptandra, Pomederris, Spyridium, Trymalium | see Australian plants |
| Rosaceae | Dryas (arctic and alpine herbs), Cercocarpus*, Purshia* *VAM also |
Hoeppel & Wollum 1971, Williams 1979, Haselwandter 1987, Kohn & Stasovski 1990. |
| Salicaceae | Populus, Salix | VAM also - Harley & Harley 1987, Brundrett et al. 1990, Wang & Qui 2006. The relative importance of ECM ad VAM varies with soil moisture - Lodge 1989. |
| Sapotaceae | Manilkara | Torti & Coley 1999 |
| Sarcolaenaceae* | Leptolaena, Sarcolaena, Schizolaena |
*Family closely related to Dipterocarpaceae and Cistaceae - Ducousso et al. 2004 |
| Tiliaceae | Tilia | Harley & Harley 1987, Brundrett et al. 1990 |
| MONOCOT | ||
| Cyperaceae | Kobresia (alpine and arctic herb) | Haselwandter 1987, Kohn & Stasovski 1990, Massicotte et al. 1998 |
- Confirmation
- Ectomycorrhizas were defined by the presence of a Hartig net confirmed by microscopic examination of cleared or sectioned roots.
- Plant family classification
- Consistent with Heywood et al. (2007) - Flowering Plant Families of the World published by the Royal Botanic Gardens, Kew.
- Highlighting
- Families with many VAM plants.
- Families with many nonmycorrhizal plants.
- Excluded families from older lists well known to have VAM, or do not meet criteria for ECM
- All ferns, Aquifoliaceae, Asteraceae, Bignoniaceae, Campanulaceae, Brassicaceae, Caprifoliaceae, Caryophyllaceae, Cornaceae, Goodenaceae, Elaeagnaceae, Lauraceae, Myricaceae, Oleaceae, Plantanaceae, Rubiaceae, Sapindaceae (Aceraceae), Saxifragaceae, Stylidiaceae, Thymeliaceae, Ulmaceae, Vitaceae.
- Myricaceae (Myrica, Comptonia)
- Despite reports that Comptonia has ECM and Myrica ECM and VAM (Rose 1980, Poole & Sylvia 1990). Most evidence suggest this is a predominantly nonmycorrhizal family (Gemma & Koske 1997, Hurd & Schwintzer 1997), with cluster roots (Berliner & Torrey 1989, Hurd & Schwintzer 1997), but some may have VAM with few arbuscules (Semones & Young 1995, Wang & Qui 2006).
- Cavendishoid associations (neotropical epiphytic Ericaceae)
- These associations seem to be more like ericoid mycorrhizas due to the presence of coils in roots,an intermittent mantle and weak hyphal growth between epidermal cells (probably not a Hartig net). See Rains et al. 2003 and Setaro et al. 2005, 2006 for more information. Reported in the genera Cavendishia, Ceratostema, Diogenesia, Disterigma, Macleania, Orthaea, Psammisia, Semiramisia, Sphyrospermum and Thibaudia.
| Examples of Ectomycorrhizal Hosts | |
 |
 |
Seeds of various species of trees in the family Dipterocarpaceae from Indonesia. |
Nothofagus cunninghamii an ECM tree from Tasmania. |
1. Dual ECM/VAM Associations
Surveys of the mycorrhizal literature have established that plants within a genus usually have the same type of mycorrhizas (ECM, VAM, etc. or remain NM) and these relationships are generally also consistent within a family (Harley & Harley 1987, Newman & Reddell 1987, Brundrett & Abbott 1991). This high correlation between plant phylogeny and mycorrhizal relationships has also been observed for families with ECM, as shown in the table above. However, Australian plants with ECM usually also have VAM and little is known about the relative importance of these associations.
Australian plants with dual VAM/ECM associations include key species used in plantation forestry belonging to the genera Casuarina, Allocasuarina (Casuarinaceae), Eucalyptus, Melaleuca (Myrtaceae) and Acacia (Mimosaceae) (Brundrett et al. 1996, Chen et al. 1999, Adams et. al. 2006). Plants with dual ECM/VAM associations are less often reported from other parts of the world (Brundrett 1991), but there are exceptions such as Alnus, Populus, Salix and Uapaca (Lodge & Wentworth 1990, Zhao Zhong, 1995; Moyersoen & Fitter, 1999). Some reports of VAM in roots of species which normally only have ECM have hyphae and vesicles, but not arbuscules (Vozzo & Hacskaylo 1974, Malloch & Malloch 1981, Harley & Harley 1987, Cázares & Trappe 1993). This may result from endophytic activity by Glomeromycotan fungi, as discussed in Section 10.
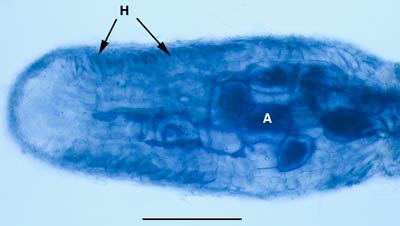 |
Dual ECM and VAM associations in Melaleuca uncinata (Myrtaceae) grown in soil from its natural habitat. Arbuscules (A) and hyphae occur in the cortex of this root tip, which also has a mantle and Hartig net (H).
|
E. Terminology
- Mantle
- layers of fungal hyphae covering the root surface.
- Hartig net
- Labyrinthine network of specialised fungus hyphae, with frequent branching (or wall ingrowths) that forms a layer between the walls of adjacent root epidermal or cortex cells. This is considered to be the major site of nutrient exchange between the fungus and host plant.
- Heterorhizy
- Root system with distinct long and short elements, resulting from reduced longitudinal growth by fine laterals.
- Short roots
- These are roots with ECM with reduced apical growth and more frequent branching, resulting in heterorhizy.
- Long roots
- The lateral roots which bear ECM short roots. These often undergo early secondary growth.
- Dichotomous branching
- This is a distinctive form of branching of the ECM short roots of some Gymnosperm trees (e.g. Pinus species). These bifurcations result in two equal branches and may result in a cluster of branches with an even number of root tips.
- Pinnate branching
- Also called sympodial branching, is unequal branching of mycorrhizal lateral roots with perpendicular side branches.
- Soil hyphae
- These are also known as extraradical or external hyphae, mycelia or the fungus thallus. They extend outwards from the fungal mantle into soil to initiate mycorrhizal associations, acquire soil nutrients, etc.
- Mycelial strands and rhizomorphs
- Interwoven hyphae that function as transport conduits and spread the association. Rhizomorphs contain specialised types of hyphae.
- Fruit bodies
- These are also called sporocarps, basidiocarps, ascocarps, mushrooms, truffles, etc. They are relatively large reproductive structures formed by Basidiomycetes or Ascomycetes which form sexual basidiospores or ascospores respectively. These develop from primordia produced by the mycelial system.
- Sclerotia
- Storage structures produced in soil by some fungi, comprised of compact fungal tissue, which is often highly melanised.
- Other spores
- Small asexual spores (conidia) which function as propagules, may be produced by some mycorrhizal fungi.
Version 2 © Mark Brundrett 2008